PRIMACARE- calcium ascorbate dihydrate, cholecalciferol, dl-a tocopheryl acetate, riboflavin, niacinamide, folate, biotin, calcium, ferrous asparto glycinate, potassium iodide, magnesium oxide, zinc bisglycinate chelate, alpha linoleic acid, dha, epa pill
PrimaCare by
Drug Labeling and Warnings
PrimaCare by is a Prescription medication manufactured, distributed, or labeled by Avion Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
DESCRIPTION: PrimaCare™ is a prescription prenatal/postnatal multivitamin/mineral/essential fatty acid softgel. Each softgel is purple in color and imprinted with “PRIMA” on one side and blank on the other.'
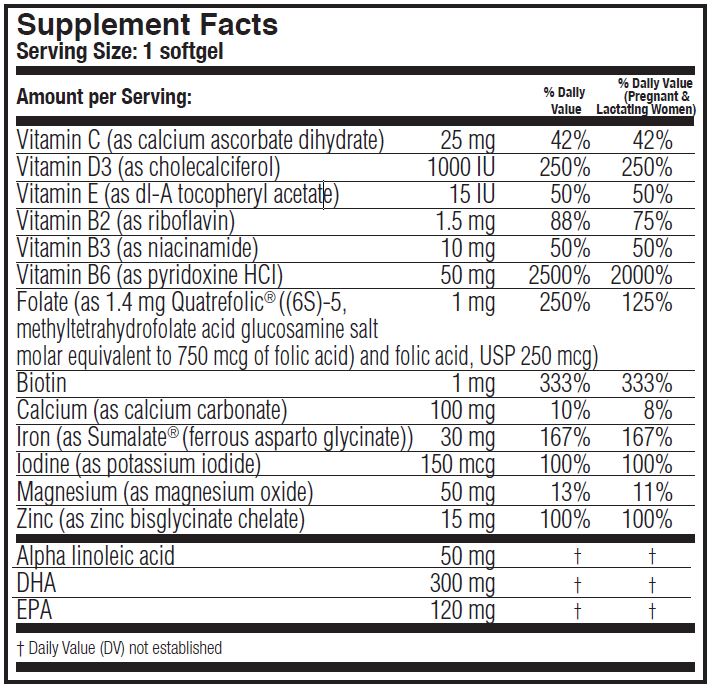
PrimaCare™ contains flaxseed oil, fish oil and soy.
OTHER INGREDIENTS: Gelatin capsule (bovine gelatin, sorbitol, glycerin, purified water, titanium dioxide, FD&C Red #40, caramel coloring, FD&C Blue #1), yellow beeswax and soy lecithin.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
BOXED WARNING
(What is this?)
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided n patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
- PRECAUTIONS
- BOXED WARNING (What is this?)
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
HOW SUPPLIED: Bottles of 30 softgels (75854-322-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
-
STORAGE AND HANDLING
STORAGE: Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent #
7,947,662 CAS# 1181972-37-1 Sumalate® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 6,716,814, 8,007,846 and 8,425,956. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRIMACARE
calcium ascorbate dihydrate, cholecalciferol, dl-a tocopheryl acetate, riboflavin, niacinamide, folate, biotin, calcium, ferrous asparto glycinate, potassium iodide, magnesium oxide, zinc bisglycinate chelate, alpha linoleic acid, dha, epa pillProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75854-322 Route of Administration oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM ASCORBATE (UNII: 183E4W213W) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 25 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 1000 [iU] ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) ALPHA-TOCOPHEROL ACETATE 15 [iU] riboflavin (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) riboflavin 1.5 mg niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) niacinamide 10 mg PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 50 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg Biotin (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) Biotin 1 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 100 mg potassium iodide (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 150 ug magnesium oxide (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 50 mg ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 15 mg LINOLEIC ACID (UNII: 9KJL21T0QJ) (LINOLEIC ACID - UNII:9KJL21T0QJ) LINOLEIC ACID 50 mg OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3 FATTY ACIDS 420 mg Inactive Ingredients Ingredient Name Strength FERROUS ASPARTO GLYCINATE (UNII: H7426RGB3L) 30 mg GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CARNAUBA WAX (UNII: R12CBM0EIZ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color purple Score no score Shape capsule Size 24mm Flavor Imprint Code PRIMA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75854-322-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/23/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/23/2017 Labeler - Avion Pharmaceuticals, LLC (965450542) Registrant - Avion Pharmaceuticals, LLC (965450542) Establishment Name Address ID/FEI Business Operations Avion Pharmaceuticals, LLC 965450542 manufacture(75854-322)
Trademark Results [PrimaCare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRIMACARE 88414718 not registered Live/Pending |
Aliera Healthcare, Inc. 2019-05-03 |
 PRIMACARE 88234123 not registered Live/Pending |
AVION PHARMACEUTICALS, LLC 2018-12-18 |
 PRIMACARE 86889995 5237812 Dead/Cancelled |
AVION PHARMACEUTICALS, LLC 2016-01-28 |
 PRIMACARE 78637492 3235953 Live/Registered |
NEXTCARE TEXAS LLC 2005-05-25 |
 PRIMACARE 75723732 2582817 Dead/Cancelled |
LUMARA HEALTH IP LTD. 1999-06-08 |
 PRIMACARE 74213028 not registered Dead/Abandoned |
Universal Converter, Inc. 1991-10-11 |
 PRIMACARE 74020733 1673743 Dead/Cancelled |
General Health Services Corporation 1990-01-22 |
 PRIMACARE 73415104 1279721 Dead/Cancelled |
Murray Salk, Inc. 1983-02-28 |
 PRIMACARE 73252778 1191399 Dead/Cancelled |
Primacare, Inc. 1980-03-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
