TRIMETHOBENZAMIDE HYDROCHLORIDE capsule
Trimethobenzamide Hydrochloride by
Drug Labeling and Warnings
Trimethobenzamide Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Sun Pharmaceutical Industries, Inc., Frontida Biopharm Inc., Alkaloida Chemical Company Zrt.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRIMETHOBENZAMIDE HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for TRIMETHOBENZAMIDE HYDROCHLORIDE CAPSULES.
TRIMETHOBENZAMIDE HYDROCHLORIDE capsules, for oral use
Initial U.S. Approval: 1974INDICATIONS AND USAGE
Trimethobenzamide hydrochloride capsules are an antiemetic indicated in adults for the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis. (1)
Limitation of Use:
Trimethobenzamide hydrochloride capsules are not recommended for use in pediatric patients due to the risk of extrapyramidal signs and symptoms and other serious central nervous system (CNS) effects and the risk of exacerbation of the underlying disease in pediatric patients with Reye’s syndrome or other hepatic impairment. (1, 8.4)
DOSAGE AND ADMINISTRATION
The recommended adult dosage is 300 mg orally three or four times daily. (2.1)
Geriatric patients and/or patients with renal impairment (creatinine clearance 70 mL/min/1.73m2 or less): Reduce the daily dosage by increasing the dosing interval; monitor renal function. (2.2, 8.5, 8.6)
Select the lowest effective daily dosage and adjust as needed based upon therapeutic response and tolerability. (2.1, 2.2)
DOSAGE FORMS AND STRENGTHS
Capsule: 300 mg of trimethobenzamide hydrochloride (3)
CONTRAINDICATIONS
Known hypersensitivity to trimethobenzamide (4)
WARNINGS AND PRECAUTIONS
- Acute Dystonic Reactions and Other Extrapyramidal Symptoms (EPS): Depending on the severity of symptoms, reduce the dosage or discontinue the drug. Treat acute dystonic reactions with anticholinergics. Avoid trimethobenzamide hydrochloride capsules in patients receiving other drugs that are likely to cause EPS. (5.1, 7.2)
- Masking of Other Serious Disorders: EPS and other CNS symptoms in patients treated with trimethobenzamide hydrochloride capsules may be confused with CNS signs of undiagnosed primary disease (e.g., encephalopathy, metabolic imbalance, Reye’s Syndrome). If CNS symptoms occur, evaluate the risks and benefits of continuing trimethobenzamide hydrochloride tablets. (5.2, 7.2)
- Other CNS Reactions: Coma, depression of mood, disorientation, and seizures have been reported. The recent use of other drugs that cause CNS depression or EPS symptoms may also increase the risk; consider reducing the dosage or discontinuing the drug. (5.3, 7.1, 7.2)
- Hepatotoxicity: Avoid use in patients whose signs and symptoms suggest the presence of hepatic impairment. Discontinue trimethobenzamide hydrochloride capsules in patients who develop impaired liver function while on treatment. (5.4, 8.7)
- Effects on the Ability to Drive or Operate Machinery: Mental and/or physical abilities may be impaired. Concomitant use of other drugs that cause CNS depression or EPS symptoms may increase this effect; either trimethobenzamide hydrochloride capsules or the other interacting drug should be chosen, depending on the importance of the drug to the patient. (5.5, 7.1, 7.2)
ADVERSE REACTIONS
Adverse reactions include hypersensitivity reactions and Parkinson-like symptoms; blood dyscrasias, blurring of vision, coma, convulsions, depression of mood, diarrhea, disorientation, dizziness, drowsiness, headache, jaundice, muscle cramps, and opisthotonos. (6)
To report SUSPECTED ADVERSE REACTIONS, contact [Sun Pharmaceutical Industries, Inc. at 1-800-406-7984] or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Alcohol: May cause drowsiness; avoid concomitant use. (7.1)
Other Drugs that Cause CNS Depression or EPS: Either trimethobenzamide hydrochloride capsules or the other interacting drug should be chosen, depending on the importance of the drug to the patient. If CNS-acting drugs cannot be avoided, monitor patients for CNS adverse reactions. (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Adult Dosage
2.2 Dosage Adjustment for Geriatric Patients and/or Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Dystonic Reactions and Other Extrapyramidal Symptoms (EPS)
5.2 Masking of Other Serious Disorders
5.3 Other CNS Reactions
5.4 Hepatotoxicity
5.5 Effects on the Ability to Drive or Operate Machinery
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Alcohol
7.2 Other Drugs that Cause CNS Depression or EPS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Trimethobenzamide hydrochloride capsules are indicated in adults for the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
Limitation of Use:
Trimethobenzamide hydrochloride capsules are not recommended for use in pediatric patients due to the risk of extrapyramidal signs and symptoms and other serious central nervous system (CNS) effects, and the risk of exacerbation of the underlying disease in pediatric patients with Reye’s syndrome or other hepatic impairment.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Adult Dosage
The recommended adult dosage is 300 mg orally three or four times daily. Select the lowest effective daily dosage and adjust as needed based upon therapeutic response and tolerability.
2.2 Dosage Adjustment for Geriatric Patients and/or Patients with Renal Impairment
In geriatric patients and/or in patients with renal impairment (creatinine clearance 70 mL/min/1.73m2 or less), reduce the daily dosage of trimethobenzamide hydrochloride capsules by increasing the dosing interval and adjust as needed based upon therapeutic response and tolerability. Monitor renal function [see Use in Specific Populations (8.5, 8.6)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Trimethobenzamide hydrochloride capsules are contraindicated in patients with known hypersensitivity to trimethobenzamide [see Adverse Reactions (6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Dystonic Reactions and Other Extrapyramidal Symptoms (EPS)
Extrapyramidal symptoms (EPS), manifested primarily as acute dystonic reactions, may occur with trimethobenzamide hydrochloride capsules. Dystonic reactions may include sudden onset of muscular spasms, especially in the head and neck or opisthotonos. Other EPS include laryngospasm, dysphagia, and oculogyric crisis. Involuntary spasms of the tongue and mouth may lead to difficulty in speaking and swallowing. Anticholinergic drugs can be used to treat acute dystonic reactions.
EPS may also include akathisia, restlessness, akinesia, and other parkinsonian-like symptoms (e.g., tremor). Depending on the severity of symptoms, reduce the daily dosage of trimethobenzamide hydrochloride capsules by increasing the dosing interval or discontinue trimethobenzamide hydrochloride capsules [see Dosage and Administration (2.1)].
Avoid trimethobenzamide hydrochloride capsules in patients receiving other drugs that are likely to cause EPS (e.g. antipsychotics) [see Drug Interactions (7.2)].
5.2 Masking of Other Serious Disorders
EPS and other CNS symptoms which can occur in patients treated with trimethobenzamide hydrochloride capsules may be confused with CNS signs of undiagnosed primary disease (e.g., encephalopathy, metabolic imbalance, Reye’s syndrome)) [see Warnings and Precautions (5.1, 5.3)]. If CNS symptoms occur, evaluate the risks and benefits of continuing trimethobenzamide hydrochloride capsules for each patient.
5.3 Other CNS Reactions
Other serious CNS adverse reactions such as coma, depression of mood, disorientation, and seizures have been reported with trimethobenzamide hydrochloride capsules administration. The recent use of other drugs that cause CNS depression or EPS symptoms (e.g., alcohol, sedatives, hypnotics, opiates, anxiolytics, antipsychotics, and anticholinergics) may also increase the risk for these serious CNS reactions [see Warnings and Precautions (5.1, 5.5)]. Consider reducing the daily dosage of trimethobenzamide hydrochloride capsules by increasing the dosing interval or discontinuing the drug [see Dosage and Administration (2.1), Drug Interactions (7.1, 7.2)].
5.4 Hepatotoxicity
Trimethobenzamide hydrochloride capsules are potentially hepatotoxic [see Adverse Reactions (6)]. Avoid use of trimethobenzamide hydrochloride capsules in patients whose signs and symptoms suggest the presence of hepatic impairment. Discontinue trimethobenzamide hydrochloride capsules in patients who develop impaired liver function while taking trimethobenzamide hydrochloride capsules.
5.5 Effects on the Ability to Drive or Operate Machinery
Trimethobenzamide hydrochloride capsules can cause drowsiness and may impair the mental and/or physical abilities required for the performance of hazardous tasks such as driving a motor vehicle or operating machinery [see Warnings and Precautions (5.1, 5.3)]. Concomitant use of other drugs that cause CNS depression or EPS symptoms (e.g., alcohol, sedatives, hypnotics, opiates, anxiolytics, antipsychotics, and anticholinergics) may increase this effect. Either trimethobenzamide hydrochloride capsules or the other interacting drug should be chosen, depending on the importance of the drug to the patient [Drug Interactions (7.1, 7.2)]. Inform patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that trimethobenzamide hydrochloride capsules do not affect them adversely.
-
6 ADVERSE REACTIONS
The following adverse reactions from voluntary reports or clinical studies have been reported with trimethobenzamide. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous system disorders: Parkinson-like symptoms, coma, convulsions, opisthotonos, dizziness, drowsiness, headache, [see Warnings and Precautions (5.1, 5.2, 5.3)]
Psychiatric disorders: disorientation, depression of mood
Eye disorders: blurred vision
Hematologic disorders: blood dyscrasias
Hepatobiliary disorders: jaundice [see Warnings and Precautions(5.4)]
Immune system disorders: hypersensitivity, including angioedema and allergic-type skin reactions
Gastrointestinal disorders: diarrhea
Musculoskeletal disorders: muscle cramps
-
7 DRUG INTERACTIONS
7.1 Alcohol
Alcohol may increase the CNS depressant effects of trimethobenzamide hydrochloride capsules and may cause drowsiness [see Warnings and Precautions (5.3, 5.5)]. Avoid concomitant use of trimethobenzamide hydrochloride capsules with alcohol.
7.2 Other Drugs that Cause CNS Depression or EPS
The concurrent use of trimethobenzamide hydrochloride capsules with other drugs that cause CNS depression or EPS (e.g., sedatives, hypnotics, opiates, anxiolytics, antipsychotics, and anticholinergics, may potentiate the effects of trimethobenzamide hydrochloride capsules [see Warnings and Precautions (5.1, 5.2, 5.3, 5.5)]. Either trimethobenzamide hydrochloride capsules or the other interacting drug should be chosen, depending on the importance of the drug to the patient. If CNS-acting drugs cannot be avoided, monitor patients for CNS adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data with trimethobenzamide in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. No adverse developmental effect was observed in animal reproduction studies with administration of trimethobenzamide hydrochloride during organogenesis in pregnant rats at doses 0.16 and 0.8 times the recommended human dose (RHD) and in pregnant rabbits at doses 1.6 times the RHD [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Reproduction studies with trimethobenzamide hydrochloride were conducted in rats and rabbits following administration of trimethobenzamide hydrochloride during organogenesis and no adverse developmental effect was observed in either species. The only effects observed were an increased percentage of embryonic resorptions or stillborn pups in rats administered 20 mg/kg and 100 mg/kg (0.16 and 0.8 times the RHD of 1200 mg/day, based on body surface area) and increased resorptions in rabbits receiving 100 mg/kg (1.6 times the RHD of 1200 mg/day, based on body surface area). In each study, these adverse effects were attributed to one or two dams.
8.2 Lactation
Risk Summary
There is no information on the presence of trimethobenzamide in human milk, the effects of trimethobenzamide hydrochloride capsules on the breastfed infant or the effects of trimethobenzamide hydrochloride capsules on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of trimethobenzamide hydrochloride capsules to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for trimethobenzamide hydrochloride capsules and any potential adverse effects on the breastfed infant from trimethobenzamide hydrochloride capsules or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of trimethobenzamide hydrochloride capsules in pediatric patients have not been established.
Trimethobenzamide hydrochloride capsules are not recommended for use in pediatric patients due to the risk of EPS and other serious CNS effects, and the risk of exacerbation of underlying disease in pediatric patients with Reye’s Syndrome, or other hepatic impairment [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
8.5 Geriatric Use
Clinical studies of trimethobenzamide did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Although there are studies reported in the literature that included geriatric patients 65 years and older with younger patients, it is not known if there are differences in efficacy or safety parameters for geriatric and non-geriatric patients treated with trimethobenzamide hydrochloride capsules.
Trimethobenzamide is excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because geriatric patients are more likely to have decreased renal function, reduce the daily dosage of trimethobenzamide hydrochloride capsules by increasing the dosing interval and adjust as needed based upon therapeutic response and tolerability. Monitor renal function [see Dosage and Administration (2.2), Use in Specific Populations (8.6)].
8.6 Renal Impairment
Trimethobenzamide is eliminated by renal excretion [see Clinical Pharmacology (12.3)]. In patients with renal impairment (creatinine clearance 70 mL/min/1.73m2 or less), reduce the daily dosage by increasing the dosing interval and adjust as needed based upon therapeutic response and tolerability. Monitor renal function [see Dosage and Administration (2.2)].
8.7 Hepatic Impairment
Avoid trimethobenzamide hydrochloride capsules in patients whose signs and symptoms suggest the presence of hepatic impairment due to the risk of hepatotoxicity [see Warnings and Precautions (5.4)]. Discontinue trimethobenzamide hydrochloride capsules in patients who develop impaired liver function while taking trimethobenzamide hydrochloride capsules.
-
11 DESCRIPTION
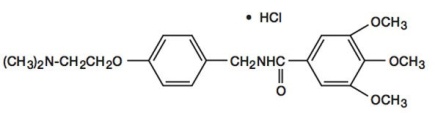
Chemically, trimethobenzamide HCl is N-[p-[2-(dimethylamino)ethoxy]benzyl]-3,4,5-trimethoxybenzamide monohydrochloride. It has a molecular weight of 424.93 and the following structural formula:
Each capsule for oral use contains trimethobenzamide hydrochloride equivalent to 300 mg.
Inactive Ingredients: FDA/E172 Red Iron Oxide, gelatin, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and titanium dioxide. The imprinting ink contains D&C Yellow #10 Lake, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, Iron Oxide Black, propylene glycol and shellac glaze.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of trimethobenzamide as determined in animals is obscure, but may involve the chemoreceptor trigger zone (CTZ), an area in the medulla oblongata through which emetic impulses are conveyed to the vomiting center; direct impulses to the vomiting center apparently are not similarly inhibited. In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of trimethobenzamide in healthy adult subjects were compared when trimethobenzamide hydrochloride capsules was administered as a 300 mg oral capsule or a 200 mg (100 mg/mL) intramuscular injection. The time to reach maximum plasma concentration (Tmax) was about 30 minutes after intramuscular injection compared to about 45 minutes after oral capsule administration. The plasma concentration-time profile of trimethobenzamide was similar between the two formulations.
Elimination
The mean elimination half-life of trimethobenzamide is 7 to 9 hours.
Metabolism
The major pathway of trimethobenzamide metabolism is through oxidation resulting in the formation of trimethobenzamide N-oxide metabolite. The pharmacologic activity of this major metabolite has not been evaluated.
Excretion
Between 30 to 50% of a single dose in humans is excreted unchanged in the urine within 48 to 72 hours.
Specific Populations
Sex
Systemic exposure to trimethobenzamide was similar between men (N=40) and women (N=28). Following a single 300 mg capsule oral administration, the respective mean (SD) Cmax of trimethobenzamide were 3.5 (1.1) and 4.2 (1.6) micrograms/mL in male and female subjects. The respective mean (SD) of AUC0-∞ of trimethobenzamide were 10 (2.7) and 10.4 (2.7) micrograms×hour/mL in male and female subjects.
Race
Pharmacokinetics appeared to be similar for Caucasians (N=53) and African Americans (N=12). Following a single 300 mg capsule oral administration, the respective mean (SD) Cmax of trimethobenzamide was 3.8 (1.3) micrograms/mL in Caucasians and 3.9 (1.7) micrograms/mL in African Americans. The respective mean (SD) AUC0-∞ of trimethobenzamide was 10.4 (2.8) micrograms×hour/mL in Caucasians and 9.8 (2.5) micrograms×hour/mL in African Americans.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Trimethobenzamide hydrochloride capsules, USP are supplied as follows:
Trimethobenzamide hydrochloride capsules, USP 300 mg, swedish orange opaque, imprinted MUTUAL over 401 on both the body and cap.
Bottles of 30
NDC: 53489-376-07
Bottles of 60
NDC: 53489-376-06
Bottles of 100
NDC: 53489-376-01
Bottles of 250
NDC: 53489-376-03
Bottles of 500
NDC: 53489-376-05
Bottles of 1000
NDC: 53489-376-10
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature]
DISPENSE IN TIGHT, LIGHT-RESISTANT CONTAINER.
-
17 PATIENT COUNSELING INFORMATION
Inform patients that trimethobenzamide hydrochloride capsules can cause serious adverse reactions. Instruct patients to discontinue trimethobenzamide hydrochloride capsules and contact a healthcare provider immediately if the following serious reactions occur:
- Acute Dystonic Reactions and Other Extrapyramidal Symptoms [see Warnings and Precautions (5.1)]
- Other CNS Reactions [see Warnings and Precautions (5.2, 5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
Effects on the Ability to Drive or Operate Machinery
Advise patients that trimethobenzamide hydrochloride capsules can cause drowsiness and may impair their judgment, thinking, or motor skills required for tasks such as driving a motor vehicle or operating machinery. Inform patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that trimethobenzamide hydrochloride capsules do not affect them adversely. [see Warnings and Precautions (5.5)]
Drug Interactions
Inform patients that use of alcohol or concomitant treatment with other CNS-acting drugs can precipitate or worsen CNS depression and/or EPS [see Drug Interactions (7.1, 7.2)]. Instruct patients avoid alcohol and to tell their health care providers when they start taking any concomitant medication.
- SPL UNCLASSIFIED SECTION
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRIMETHOBENZAMIDE HYDROCHLORIDE
trimethobenzamide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 53489-376 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trimethobenzamide Hydrochloride (UNII: WDQ5P1SX7Q) (Trimethobenzamide - UNII:W2X096QY97) Trimethobenzamide Hydrochloride 300 mg Inactive Ingredients Ingredient Name Strength ferric oxide red (UNII: 1K09F3G675) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) magnesium stearate (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) titanium dioxide (UNII: 15FIX9V2JP) D&C yellow no. 10 (UNII: 35SW5USQ3G) Aluminum oxide (UNII: LMI26O6933) FD&C blue no. 1 (UNII: H3R47K3TBD) FD&C blue no. 2 (UNII: L06K8R7DQK) FD&C red no. 40 (UNII: WZB9127XOA) ferrosoferric oxide (UNII: XM0M87F357) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Sodium Starch Glycolate Type A Potato (UNII: 5856J3G2A2) Product Characteristics Color ORANGE (Swedish Orange Opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Mutual;401 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53489-376-07 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 2 NDC: 53489-376-06 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 3 NDC: 53489-376-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 4 NDC: 53489-376-03 250 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 5 NDC: 53489-376-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 6 NDC: 53489-376-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/28/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076570 08/28/2003 Labeler - Sun Pharmaceutical Industries, Inc. (146974886) Establishment Name Address ID/FEI Business Operations Frontida Biopharm Inc. 080243260 MANUFACTURE(53489-376) Establishment Name Address ID/FEI Business Operations Alkaloida Chemical Company Zrt. 643611692 MANUFACTURE(53489-376)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.