ROBAFEN DM- dextromethorphan hydrobromide, guaifenesin solution
robafen dm by
Drug Labeling and Warnings
robafen dm by is a Otc medication manufactured, distributed, or labeled by Preferred Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each 20 mL)
- Purposes
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
-
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- keep dosing cup with product
- mL = milliliter
- this adult product is not intended for use in children under 12 years of age
age
dose
adults and children 12 years and over
20 mL every 4 hours
children under 12 years
do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
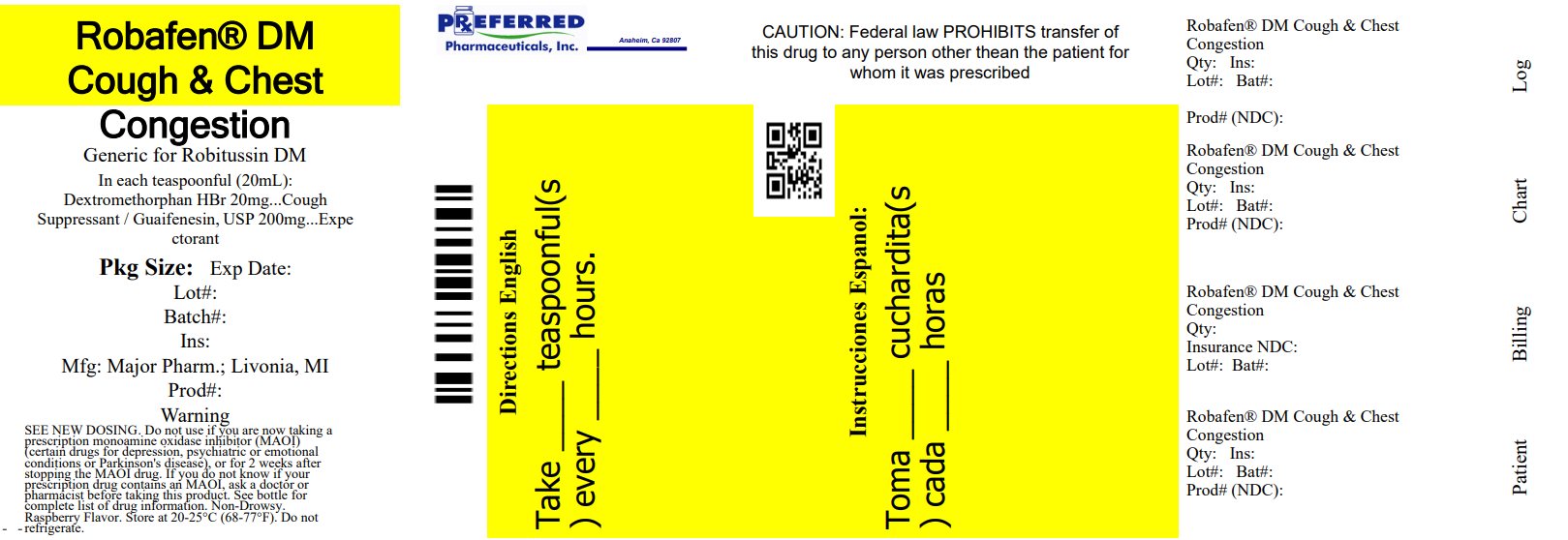
Package/Label Principal Display Panel
MAJOR®
Relabeled By: Preferred Pharmaceuticals Inc.
Compare to Robitussin® Cough + Chest Congestion DM active ingredients
Robafen® DM Cough and Chest Congestion
Dextromethorphan HBr, USP 20 mg
Guaifenesin, USP 200 mg
Cough Suppressant
Expectorant
Controls Cough
Relieves Chest Congestion
Thins and Loosens Mucus
SEE NEW DOSING
Raspberry Flavor
Non-Drowsy
For Adults Ages 12 and Over
4 FL. OZ. (118 mL)
-
INGREDIENTS AND APPEARANCE
ROBAFEN DM
dextromethorphan hydrobromide, guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68788-8268(NDC:0904-7223) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color RED Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68788-8268-1 1 in 1 CARTON 09/23/2022 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part341 09/23/2022 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8268)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.