RELIEF- sodium phosphate, monobasic and sodium phosphate, dibasic, heptahydrate enema
RELIEF by
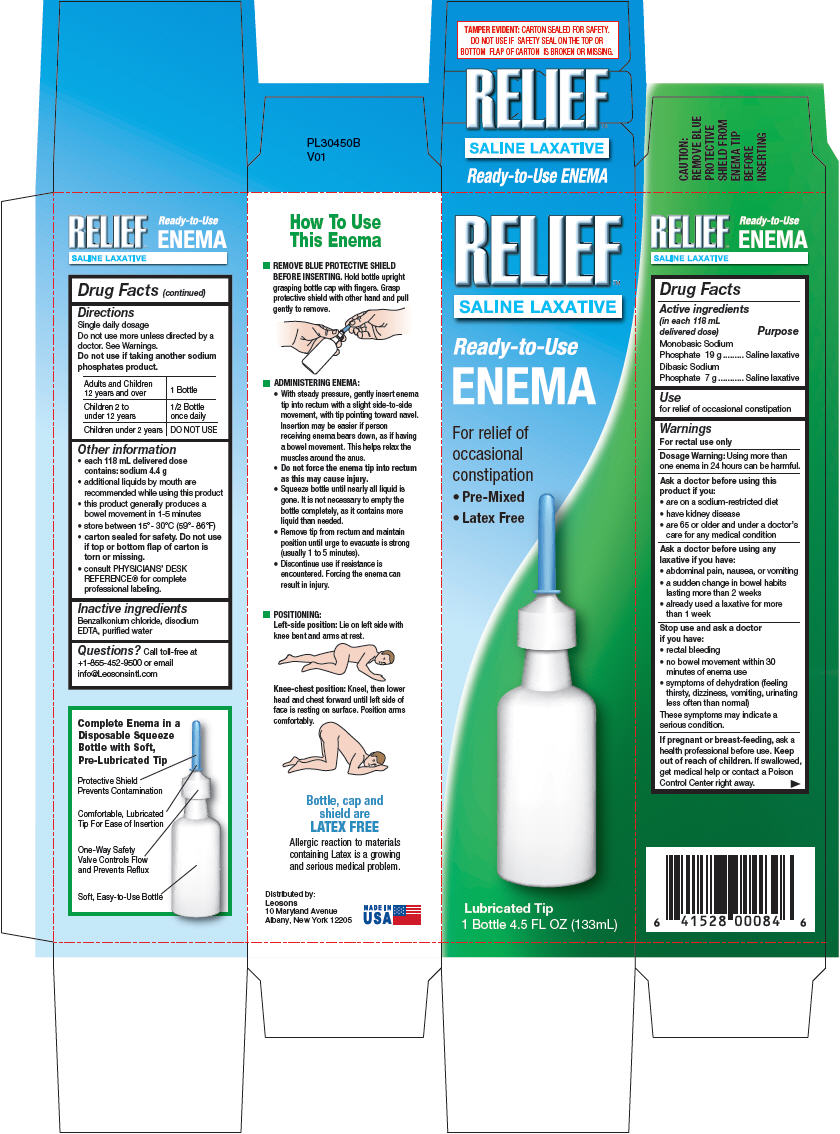
Drug Labeling and Warnings
RELIEF by is a Otc medication manufactured, distributed, or labeled by Leosons Overseas Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
For rectal use only
Ask a doctor before using this product if you
- are on a sodium-restricted diet
- have kidney disease
- are 65 or older and under a doctor's care for any medical condition
Ask a doctor before using any laxative if you have
- abdominal pain, nausea, or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
-
Directions
Single daily dosage
Do not use more unless directed by a doctor. See Warnings.
Do not use if taking another sodium phosphates product.
Adults and Children 12 years and over 1 Bottle Children 2 to under 12 years 1/2 Bottle once daily Children under 2 years DO NOT USE -
Other information
- each 118 mL delivered dose contains: sodium 4.4 g
- additional liquids by mouth are recommended while using this product
- this product generally produces a bowel movement in 1-5 minutes
- store between 15°- 30°C (59°- 86°F)
- carton sealed for safety. Do not use if top or bottom flap of carton is torn or missing.
- consult PHYSICIANS' DESK REFERENCE® for complete professional labeling.
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 133 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
RELIEF
sodium phosphate, monobasic and sodium phosphate, dibasic, heptahydrate enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69626-0084 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC 19 g in 133 mL SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 7 g in 133 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69626-0084-6 1 in 1 CARTON 1 133 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 02/09/2015 Labeler - Leosons Overseas Corp (148605470)
Trademark Results [RELIEF]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RELIEF 98244926 not registered Live/Pending |
Relief Innovations LLC 2023-10-28 |
 RELIEF 97761768 not registered Live/Pending |
The Ureteral Stent Company 2023-01-20 |
 RELIEF 97206537 not registered Live/Pending |
Relief S.r.l. 2022-01-06 |
 RELIEF 90252354 not registered Live/Pending |
Relief Technologies, Inc 2020-10-13 |
 RELIEF 88879542 not registered Live/Pending |
Minnesota Hemp Farms, Inc. 2020-04-20 |
 RELIEF 88657515 not registered Live/Pending |
SKIN ALCHEMY LLC 2019-10-16 |
 RELIEF 88500058 not registered Live/Pending |
SKIN ALCHEMY LLC 2019-07-03 |
 RELIEF 88478409 not registered Live/Pending |
Cyphi, LLC 2019-06-18 |
 RELIEF 88359405 not registered Live/Pending |
GSW Creative Corporation 2019-03-27 |
 RELIEF 87880995 not registered Live/Pending |
GSW Creative Corporation 2018-04-17 |
 RELIEF 87617809 not registered Dead/Abandoned |
Stoller Enterprises, Inc. 2017-09-21 |
 RELIEF 87571638 not registered Dead/Abandoned |
Stoller Enterprises, Inc. 2017-08-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.