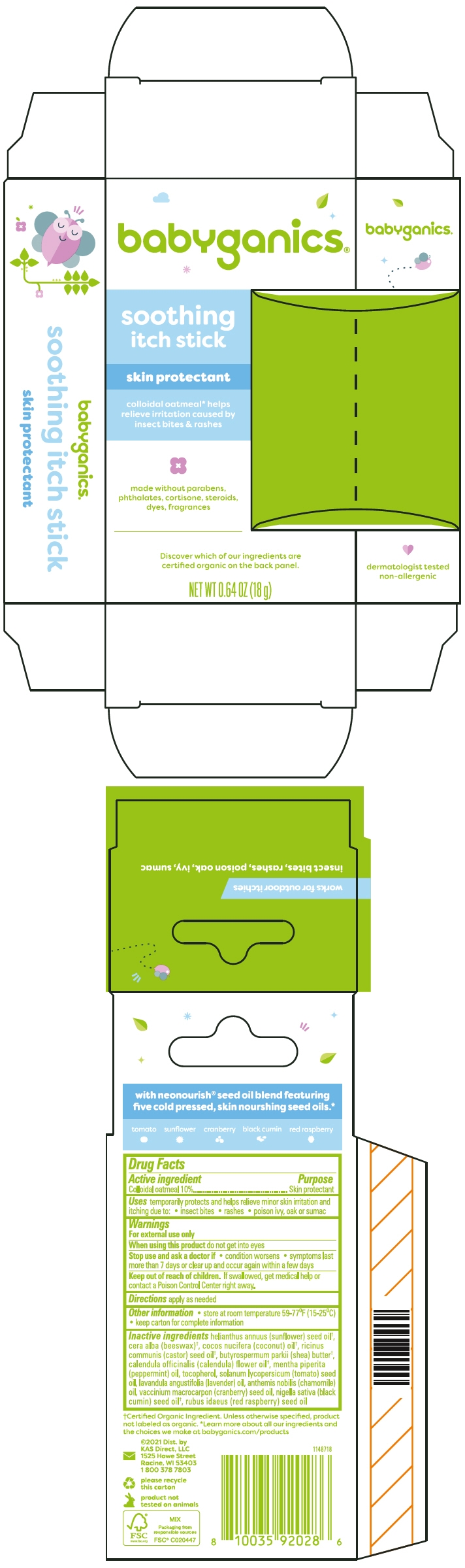
BABYGANICS SOOTHING ITCH- oatmeal stick
Babyganics Soothing Itch by
Drug Labeling and Warnings
Babyganics Soothing Itch by is a Otc medication manufactured, distributed, or labeled by KAS Direct, LLC, Cosmetic Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
helianthus annuus (sunflower) seed oil1, cera alba (beeswax)1, cocos nucifera (coconut) oil1, ricinus communis (castor) seed oil1, butyrospermum parkii (shea) butter1, calendula officinalis (calendula) flower oil1, mentha piperita (peppermint) oil, tocopherol, solanum lycopersicum (tomato) seed oil, lavandula angustifolia (lavender) oil, anthemis nobilis (chamomile) oil,vaccinium macrocarpon (cranberry) seed oil, nigella sativa (black cumin) seed oil1, rubus idaeus (red raspberry) seed oil
- 1 Certified Organic Ingredient. Unless otherwise specified, product not labeled as organic.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 18 g Stick Carton
-
INGREDIENTS AND APPEARANCE
BABYGANICS SOOTHING ITCH
oatmeal stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59062-3301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength SUNFLOWER SEED (UNII: R9N3379M4Z) WHITE WAX (UNII: 7G1J5DA97F) COCONUT OIL (UNII: Q9L0O73W7L) CASTOR OIL (UNII: D5340Y2I9G) SHEA BUTTER (UNII: K49155WL9Y) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) PEPPERMINT OIL (UNII: AV092KU4JH) Tocopherol (UNII: R0ZB2556P8) TOMATO SEED OIL (UNII: 7N87T9C06T) LAVENDER OIL (UNII: ZBP1YXW0H8) CHAMAEMELUM NOBILE FLOWER OIL (UNII: UB27587839) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) NIGELLA SATIVA SEED OIL (UNII: CS4U38E731) RASPBERRY SEED OIL (UNII: 9S8867952A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59062-3301-1 1 in 1 CARTON 09/01/2024 1 18 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M016 09/01/2024 Labeler - KAS Direct, LLC (002764605) Establishment Name Address ID/FEI Business Operations Cosmetic Solutions 807907928 MANUFACTURE(59062-3301)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.