Siltussin SA (Guaifenesin Liquid)
Siltussin SA by
Drug Labeling and Warnings
Siltussin SA by is a Otc medication manufactured, distributed, or labeled by REMEDYREPACK INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SILTUSSIN SA- guaifenesin liquid
REMEDYREPACK INC.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Siltussin SA (Guaifenesin Liquid)
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- repeat dose every 4 hours
| adults and children 12 years and over
| 2-4 teaspoonfuls (TSP)
|
| children under 12 years | DO NOT USE |
Other information
Store at room temperature 20°-25°C (68°-77°F).
Do not accept if imprinted tamper evident safety seal around cap is broken or missing.
Inactive ingredients
citric acid, D&C yellow no. 10, FD&C blue no. 1, FD&C red no. 40, glycerin, menthol, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol, strawberry flavor.
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
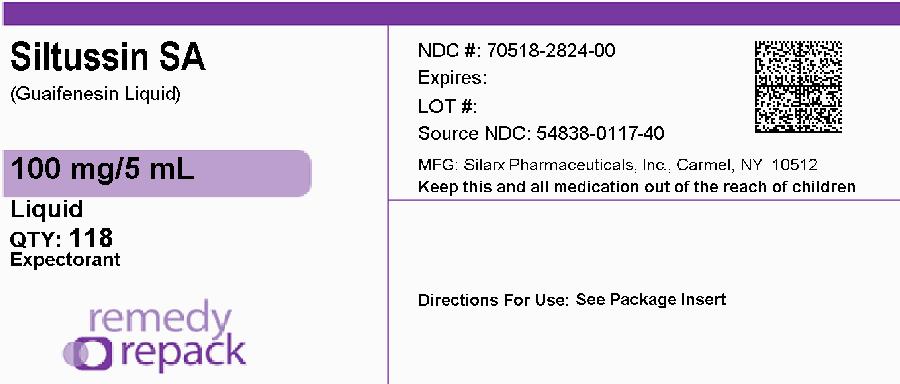
DRUG: Siltussin SA
GENERIC: Guaifenesin
DOSAGE: LIQUID
ADMINSTRATION: ORAL
NDC: 70518-2824-0
NDC: 70518-2824-1
NDC: 70518-2824-2
FLAVOR: STRAWBERRY
PACKAGING: 118 mL in 1 BOTTLE, PLASTIC
PACKAGING: 10 in 1 BOX
PACKAGING: 20 mL in 1 CUP, UNIT DOSE TYPE 0
ACTIVE INGREDIENT(S):
- GUAIFENESIN 100mg in 5mL
INACTIVE INGREDIENT(S):
- ANHYDROUS CITRIC ACID
- D&C YELLOW NO. 10
- FD&C BLUE NO. 1
- FD&C RED NO. 40
- GLYCERIN
- MENTHOL
- PROPYLENE GLYCOL
- WATER
- SACCHARIN SODIUM
- SODIUM BENZOATE
- SORBITOL


| SILTUSSIN SA
guaifenesin liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.