LANSOPRAZOLE capsule, delayed release
Lansoprazole by
Drug Labeling and Warnings
Lansoprazole by is a Otc medication manufactured, distributed, or labeled by Wockhardt USA LLC., Wockhardt Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- USE
-
WARNINGS
Allergy alert: Do not use if you are allergic to lansoprazole
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- liver disease
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking
- warfarin (blood-thinning medicine)
- prescription antifungal or anti-yeast medicines
- digoxin (heart medicine)
- theophylline (asthma medicine)
- tacrolimus (immune system medicine)
- atazanavir (medicine for HIV infection)
- OTC - KEEP OUT OF REACH OF CHILDREN
-
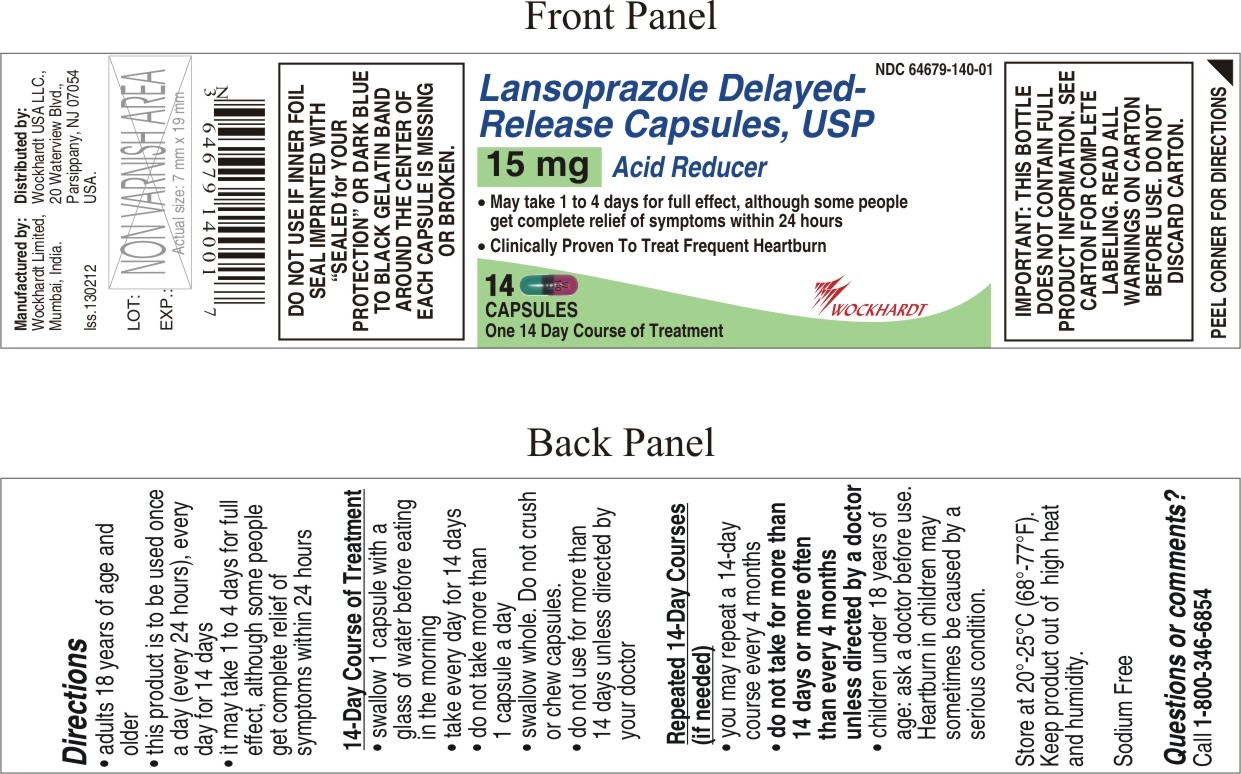
DIRECTIONS
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- Other information
- INACTIVE INGREDIENT
- OTC - QUESTIONS
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANSOPRAZOLE
lansoprazole capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64679-140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM CARBONATE (UNII: 0E53J927NA) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) Product Characteristics Color PINK (Opaque dark pink cap) , GREEN (Opaque dark green body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code W;140 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64679-140-00 4500 in 1 POUCH; Type 0: Not a Combination Product 05/18/2012 2 NDC: 64679-140-01 1 in 1 CARTON 05/18/2012 2 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 64679-140-08 2 in 1 CARTON 05/18/2012 3 14 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 64679-140-09 3 in 1 CARTON 05/18/2012 4 14 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 64679-140-07 1 in 1 CARTON 05/18/2012 5 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC: 64679-140-10 2 in 1 CARTON 05/18/2012 6 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC: 64679-140-11 3 in 1 CARTON 05/18/2012 7 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202727 05/18/2012 Labeler - Wockhardt USA LLC. (170508365) Registrant - Wockhardt USA LLC. (170508365) Establishment Name Address ID/FEI Business Operations Wockhardt Limited 916489953 ANALYSIS(64679-140) , LABEL(64679-140) , MANUFACTURE(64679-140) , PACK(64679-140)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.