DEMECLOCYCLINE HYDROCHLORIDE tablet, film coated
Demeclocycline Hydrochloride by
Drug Labeling and Warnings
Demeclocycline Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Epic Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Full Prescribing Information
To reduce the development of drug-resistant bacteria and maintain the effectiveness of demeclocycline hydrochloride tablets and other antibacterial drugs, demeclocycline hydrochloride tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
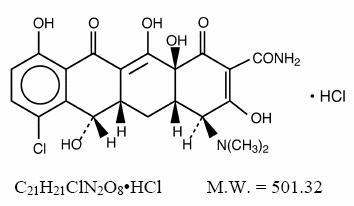
Demeclocycline hydrochloride, USP is an antibiotic isolated from a mutant strain of Streptomyces aureofaciens. Chemically it is 7- Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12, 12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride.
Its structural formula is:
Each film-coated tablet, for oral administration contains 150 mg or 300 mg of demeclocycline hydrochloride, USP and has the following inactive ingredients: carnauba wax, colloidal silicon dioxide, crospovidone, hydroxypropyl cellulose, hypromelloses, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, pregelatinized starch, talc, titanium dioxide, triacetin, D&C Red No. 27 Aluminum Lake, D&C Red No. 30 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
The absorption of demeclocycline is slower than that of tetracycline. The time to reach the peak concentration is about 4 hours. After a 150 mg oral dose of demeclocycline tablet, the mean concentrations at 1 hour and 3 hours are 0.46 and 1.22 mcg/mL (n = 6), respectively. The serum half-life ranges between 10 and 16 hours. When demeclocycline hydrochloride is given concomitantly with some dairy products, or antacids containing aluminum, calcium, or magnesium, the extent of absorption is reduced by more than 50%. Demeclocycline hydrochloride penetrates well into various body fluids and tissues. The percent of demeclocycline hydrochloride bound to plasma protein is about 40% using a dialysis equilibrium method and 90% using an ultra-filtration method. Demeclocycline hydrochloride, like other tetracyclines, is concentrated in the liver and excreted into the bile where it is found in much higher concentrations than in the blood. The rate of demeclocycline hydrochloride renal clearance (35 mL/min/1.73 m 2) is less than half that of tetracycline. Following a single 150 mg dose of demeclocycline hydrochloride in normal volunteers, 44% (n = 8) was excreted in urine and 13% and 46%, respectively, were excreted in feces in two patients within 96 hours as active drug.
Microbiology
Mechanism of Action
The tetracyclines are primarily bacteriostatic and are thought to exert their antimicrobial effect by the inhibition of protein synthesis. The tetracyclines, including demeclocycline, have a similar antimicrobial spectrum of activity against a wide range of gram-negative and gram-positive organisms.
Mechanism(s) of Resistance
Resistance to tetracyclines may be mediated by efflux, alteration in the target site of tetracycline, enzymatic inactivation, and decreased bacterial permeability to the tetracycline or a combination of these mechanisms.
Cross-Resistance
Cross-resistance between antibiotics of the tetracycline family occurs.
Demeclocycline has been shown to be active against most isolates of the following bacteria, in vitro and/or in clinical infections as described in the INDICATIONS AND USAGE section.
Gram-Positive Bacteria
Bacillus anthracis
Listeria monocytogenes
Staphylococcus aureus
Streptococcus pneumoniae
Gram-Negative Bacteria
Bartonella bacilliformis
Brucella species
Calymmatobacterum granulomatis
Campylobacter fetus
Francisella tularensis
Haemophilus ducreyi
Haemophilus influezae
Neisseria gonorrrhoeae
Vibrio cholerae
Yersinia pestis
Because many isolates of the following groups of gram-negative bacteria have been shown to be resistant to tetracyclines, culture and susceptibility testing are especially recommended:
Acinetobacter species
Enterobacter aerogenes
Escherichia coli
Klebsiella species
Shigella species
Other Microorganisms
Actinomyces israelii
Borella recurrentis
Chlamydia psittaci
Chlamydia trachomatis
Clostridium species
Entamoeba species
Fusobacterium fusiforme
Mycoplasma pneumoniae
Propionibacterium acnes
Rickettsiae
Treponema pallidium subspecies pallidum
Treponema pallidum subspecies pertenue
Ureaplasma urealyticum
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized method (broth/or agar) 1,2,3. The MIC values should be interpreted according to the criteria in Table 1.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds.
The zone size should be determined using a standardized test method. 2,4 This procedure uses paper disks impregnated with 30 mcg tetracycline to test the susceptibility of microorganisms to tetracycline. The disc diffusion interpretive criteria are provided in Table 1.
Table 1. Susceptibility Test Interpretive Criteria for Tetracycline Minimum Inhibitory Concentration
(mcg/mL)
Disk Diffusion
(zone diameters in mm)
Pathogen
S
I
R
S
I
R
Enerobacteriaceae, Acinetobacter spp.
≤4
8
>16
≥15
12 to 14
<11
Haemophilus influenzae
<2
4
>8
>29
26 to 28
<25
Neisseria gonorrhoeae
<0.25
0.5 to 1
>2
>38
31 to 37
<30
Staphylococcus aureus
≤4
8
≥16
≥19
15 to 18
≤14
S. pneumoniae (non-meningitis isolates)
≤1
2
≥4
>28
25 to 27
≤24
Bacillus anthracis
<1
--
--
--
--
--
Franciscella tularensis
<4
--
--
--
--
--
A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. A report of Intermediate indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug product is physiologically concentrated or in situations where a high dosage of the drug product can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test. 1,2,3,4 Standard tetracycline powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 30 mcg tetracycline disk, the criteria in Table 2 should be achieved.
Table 2. Acceptable Quality Control Ranges for Tetracycline *ATCC = American Type Culture Collection QC Strain
Minimum Inhibitory Concentrations (mcg/mL)
Disk Diffusion (zone diameters in mm)
Escherichia coli ATCC* 25922
0.5 to 2
18 to 25
Staphylococcus aureus ATCC 29213
0.12 to 1
-----
Staphylococcus aureus ATCC 25923
-----
24 to 30
Haemophilus influenzae ATCC 49247
4 to 32
14 to 22
Neisseria gonorrhoeae ATCC 49226
0.25 to 1
30 to 42
Streptococcus pneumoniae ATCC 49619
0.06 to 0.5
27 to 31
-
INDICATIONS AND USAGE
Demeclocycline hydrochloride tablets USP is indicated in the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions below:
Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by rickettsiae; Respiratory tract infections caused by Mycoplasma pneumoniae; Lymphogranuloma venereum due to Chlamydia trachomatis; Psittacosis (Ornithosis) due to Chlamydia psittaci; Trachoma due to Chlamydia trachomatis, although the infectious agent is not always eliminated, as judged by immunofluorescence; Inclusion conjunctivitis caused by Chlamydia trachomatis; Nongonococcal urethritis in adults caused by Ureaplasma urealyticum or Chlamydia trachomatis; Relapsing fever due to Borrelia recurrentis;
Chancroid caused by Haemophilus ducreyi;Plague due to Yersinia pestis;
Tularemia due to Francisella tularensis;
Cholera caused by Vibrio cholerae;
Campylobacter fetus infections caused by Campylobacter fetus;
Brucellosis due to Brucella species (in conjunction with streptomycin);
Bartonellosis due to Bartonella bacilliformis;
Granuloma inguinale caused by Calymmatobacterium granulomatis;
Demeclocycline hydrochloride tablets USP is indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug:
Escherichia coli;
Enterobacter aerogenes;
Shigella species;
Acinetobacter species;
Respiratory tract infections caused by Haemophilus influenzae;
Respiratory tract and urinary tract infections caused by Klebsiella species.
Demeclocycline hydrochloride tablets USP is indicated for treatment of infections caused by the following gram-positive microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug:
Upper respiratory infections caused by Streptococcus pneumoniae;
Skin and skin structure infections caused by Staphylococcus aureus.
(Note: Tetracyclines, including demeclocycline, are not the drugs of choice in the treatment of any type of staphylococcal infection.) When penicillin is contraindicated, tetracyclines, including demeclocycline hydrochloride, are alternative drugs in the treatment of the following infections:
Uncomplicated urethritis in men due to Neisseria gonorrhoeae, and for the treatment of other uncomplicated gonococcal infections; Infections in women caused by Neisseria gonorrhoeae;
Syphilis caused by Treponema pallidum subspecies pallidum;
Yaws caused by Treponema pallidum subspecies pertenue;
Listeriosis due to Listeria monocytogenes;
Anthrax due to Bacillus anthracis;
Vincent’s infection caused by Fusobacterium fusiforme;
Actinomycosis caused by Actinomyces israelii;
Clostridial diseases caused by Clostridium species.
In acute intestinal amebiasis, demeclocycline hydrochloride tablets USP may be a useful adjunct to amebicides.
In severe acne, demeclocycline hydrochloride tablets USP may be a useful adjunctive therapy.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of demeclocycline hydrochloride tablets USP and other antibacterial drugs, demeclocycline hydrochloride tablets USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
DEMECLOCYCLINE HYDROCHLORIDE, LIKE OTHER TETRACYCLINE-CLASS ANTIBIOTICS, CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY, OR IF THE PATIENT BECOMES PREGNANT WHILE TAKING THESE DRUGS, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS.
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN). This adverse reaction is more common during long-term use of the drugs but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED DURING TOOTH DEVELOPMENT UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED.
All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every six hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy. The anti-anabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis.
If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulation of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated and, if therapy is prolonged, serum level determinations of the drug may be advisable.
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Phototoxic reactions can occur in individuals taking demeclocycline, and are characterized by severe burns or exposed surfaces resulting from direct exposure of patients to sunlight during therapy with moderate or large doses of demeclocycline. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur, and treatment should be discontinued at the first evidence of erythema of the skin.
Administration of demeclocycline hydrochloride has resulted in appearance of the diabetes insipidus syndrome (polyuria, polydipsia and weakness) in some patients on long-term therapy. The syndrome has been shown to be nephrogenic, dose-dependent and reversible on discontinuance of therapy. Patients who are experiencing central nervous system symptoms associated with demeclocycline therapy should be cautioned about driving vehicles or using hazardous machinery while on demeclocycline therapy. Clostridium difficile associated with diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including demeclocycline hydrochloride, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Pseudotumor cerebri (benign intracranial hypertension) in adults has been associated with the use of tetracyclines. The usual clinical manifestations are headache and blurred vision. Bulging fontanels have been associated with the use of tetracyclines in infants. While both of these conditions and related symptoms usually resolve soon after discontinuation of the tetracycline, the possibility for permanent sequelae exists.
As with other antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate therapy should be instituted.
Incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy, when indicated.
Prescribing demeclocycline hydrochloride tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema. Concurrent use of tetracyclines with oral contraceptives may render oral contraceptives less effective (see Drug Interactions). Patients should be informed that demeclocycline hydrochloride tablets should be taken at least 1 hour before meals or 2 hours after meals (see DOSAGE AND ADMINISTRATION). Unused supplies of tetracycline antibiotics should be discarded by the expiration date. Patients who are experiencing headache, dizziness, light-headedness, vertigo, or blurred vision while on demeclocycline therapy, should be cautioned about driving vehicles or using hazardous machinery while receiving demeclocycline therapy (see WARNINGS).
Patients should be counseled that antibacterial drugs, including demeclocycline hydrochloride tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When demeclocycline hydrochloride tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed.
Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by demeclocycline hydrochloride tablets or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
In venereal diseases when coexistent syphilis is suspected, darkfield examination should be done before treatment is started and the blood serology repeated monthly for at least 4 months. In long-term therapy, periodic laboratory evaluation of organ systems, including hematopoietic, renal, and hepatic, should be performed. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with demeclocycline hydrochloride should have a follow-up serologic test for syphilis after 3 months.
Drug Interactions
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage. Since bacteriostatic drugs may interfere with the bactericidal action of penicillins, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
Concurrent use of tetracyclines with oral contraceptives may render oral contraceptives less effective.
The concurrent use of tetracyclines and methoxyflurane has been reported to result in fatal renal toxicity.
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium or magnesium, and by iron-containing preparations.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of demeclocycline hydrochloride have not been conducted. However, there has been evidence of oncogenic activity in rats in studies with the related antibiotics oxytetracycline (adrenal and pituitary tumors) and minocycline (thyroid tumors).
Although mutagenicity studies of demeclocycline hydrochloride have not been conducted, positive results in in vitro mammalian cell assays (i.e., mouse lymphoma and Chinese hamster lung cells) have been reported for related antibiotics (tetracycline hydrochloride and oxytetracycline). (See WARNINGS; ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGY.)
Demeclocycline hydrochloride had no effect on fertility when administered in the diet to male and female rats at a daily intake of 45 times the human dose.
Pregnancy
Teratogenic Effects
Pregnancy Category D
(See WARNINGS).
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has been noted in animals treated early in pregnancy.
Nursing Mothers
Tetracyclines are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from the tetracyclines, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. (See WARNINGS).
Pediatric Use
Not for use in patients younger than eight years of age. (See WARNINGS, PRECAUTIONS, General and DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
The following reactions have been reported in patients receiving tetracyclines:
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, pancreatitis, and inflammatory lesions (with monilial overgrowth) in the anogenital region, increases in liver enzymes, and hepatic toxicity have been reported rarely.
Rarely, hepatitis and liver failure have been reported. These reactions have been caused by both the oral and parenteral administration of tetracyclines.
Instances of esophageal ulcerations have been reported in patients receiving oral tetracyclines. Most of the patients were reported to have taken the medication immediately before lying down. (See DOSAGE AND ADMINISTRATION)
Skin: Maculopapular and erythematous rashes, erythema multiforme. Exfoliative dermatitis has been reported but is uncommon. Fixed drug eruptions and Stevens-Johnson syndrome have been reported rarely. Lesions occurring on the glans penis have caused balanitis. Pigmentation of the skin and mucous membranes has also been reported. Photosensitivity is discussed above. (See WARNINGS).
Renal toxicity: Acute renal failure. Rise in BUN has been reported and is apparently dose related. Nephrogenic diabetes insipidus. (See WARNINGS.)
Hypersensitivity reactions: Urticaria, angioneurotic edema, polyarthralgia, anaphylaxis, anaphylactoid purpura, pericarditis, exacerbation of systemic lupus erythematosus, lupus-like syndrome, pulmonary infiltrates with eosinophilia.
Hematologic: Hemolytic anemia, thrombocytopenia, neutropenia and eosinophilia have been reported.
CNS: Pseudotumor cerebri (benign intracranial hypertension) in adults and bulging fontanels in infants (see PRECAUTIONS, General). Dizziness, headache, tinnitus, and visual disturbances have been reported. Myasthenic syndrome has been reported rarely.
Other: When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function studies are known to occur. Very rare cases of abnormal thyroid function have been reported.
Tooth discoloration has occurred in pediatric patients less than 8 years of age (see WARNINGS), and has been reported rarely in adults.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Therapy should be continued for at least 24 to 48 hours after symptoms and fever have subsided.
Concomitant Therapy
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium, and by iron-containing preparations. Foods and some dairy products also interfere with absorption. Oral forms of tetracycline should be given at least 1 hour before or 2 hours after meals.
In Patients With Renal Impairment
(See WARNINGS). Tetracyclines should be used cautiously in patients with impaired renal function. Total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses.
In Patients With Liver Impairment
Tetracyclines should be used cautiously in patients with impaired liver function. Total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses. Administration of adequate amounts of fluid with the oral formulations of tetracyclines is recommended to wash down the drugs and reduce the risk of esophageal irritation and ulceration. (See ADVERSE REACTIONS).
-
HOW SUPPLIED
Adults
Usual Daily Dose
Four divided doses of 150 mg each or two divided doses of 300 mg each.
For Pediatric Patients Above Eight Years of Age
Usual daily dose, 7 to 13 mg per kg body weight per day, depending upon the severity of the disease, divided into two to four doses not to exceed adult dosage of 600 mg per day.
Gonorrhea patients sensitive to penicillin may be treated with demeclocycline administered as an initial oral dose of 600 mg followed by 300 mg every 12 hours for four days to a total of 3 grams.
Demeclocycline Hydrochloride Tablets USP, 150 mg, are round, biconvex, red film-coated tablets, debossed "Є" above "143" on one side and plain on the other side, and are supplied as follows:
NDC 42806-143-30 - Bottle of 30
NDC 42806-143-01 - Bottle of 100
NDC 42806-143-05 - Bottle of 500
Demeclocycline Hydrochloride Tablets USP, 300 mg, are round, biconvex, red, film-coated tablets, debossed "Є" above "144" on one side and plain on the other side, and are supplied as follows:
NDC 42806-144-30 - Bottle of 30
NDC 42806-144-48 - Bottle of 48
NDC 42806-144-01 - Bottle of 100
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense contents in a tight, light-resistant container as defined in the USP, with a child-resistant closure as required .
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGY
Hyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO 4 and methacycline; in minipigs by doxycycline, minocycline, tetracycline PO 4, and methacycline; in dogs by doxycycline and minocycline; in monkeys by minocycline.
Minocycline, tetracycline PO 4, methacycline, doxycycline, tetracycline base, oxytetracycline HCl, and tetracycline HCl were goitrogenic in rats fed a low iodine diet. This goitrogenic effect was accompanied by high radioactive iodine uptake. Administration of minocycline also produced a large goiter with high radioiodine uptake in rats fed a relatively high iodine diet.
Treatment of various animal species with this class of drugs has also resulted in the induction of thyroid hyperplasia in the following: in rats and dogs (minocycline), in chickens (chlortetracycline), and in rats and mice (oxytetracycline). Adrenal gland hyperplasia has been observed in goats and rats treated with oxytetracycline.
-
REFERENCES
1. Clinical and Laboratory Standards Institute (CLSI.) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard - 9th Edition. CLSI document M7-A9, 950 West Valley Rd. Suite 2500, Wayne, PA 19087, 2012.
2. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 22nd Informational Supplement. CLSI document M100-S22. Wayne, PA, 2012.
3. CLSI. Methods or Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria: Approved Guideline - 2nd Edition. CLSI document M45-A2, CLSI, Wayne, PA, 2011.
4. CLSI, Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard - 11th Edition. CLSI document M2-A11. Wayne, PA, 2012.
Manufactured by:
Epic Pharma, LLC
Laurelton, NY 11413
Manufactured in USA
Revised August 2015
MF143REV08/15
OE1469
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 150 mg
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 300 mg
-
INGREDIENTS AND APPEARANCE
DEMECLOCYCLINE HYDROCHLORIDE
demeclocycline hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42806-143 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEMECLOCYCLINE HYDROCHLORIDE (UNII: 29O079NTYT) (DEMECLOCYCLINE - UNII:5R5W9ICI6O) DEMECLOCYCLINE HYDROCHLORIDE 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 68401960MK) CARNAUBA WAX (UNII: R12CBM0EIZ) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C RED NO. 30 (UNII: 2S42T2808B) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYDROXYPROPYL CELLULOSE (TYPE L) (UNII: UKE75GEA7F) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) POLYDEXTROSE (UNII: VH2XOU12IE) Product Characteristics Color red Score no score Shape ROUND Size 9mm Flavor Imprint Code E143 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42806-143-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 2 NDC: 42806-143-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 3 NDC: 42806-143-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065447 02/02/2015 DEMECLOCYCLINE HYDROCHLORIDE
demeclocycline hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42806-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEMECLOCYCLINE HYDROCHLORIDE (UNII: 29O079NTYT) (DEMECLOCYCLINE - UNII:5R5W9ICI6O) DEMECLOCYCLINE HYDROCHLORIDE 300 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 68401960MK) CARNAUBA WAX (UNII: R12CBM0EIZ) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C RED NO. 30 (UNII: 2S42T2808B) D&C RED NO. 27 (UNII: 2LRS185U6K) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) HYDROXYPROPYL CELLULOSE (TYPE L) (UNII: UKE75GEA7F) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) POLYDEXTROSE (UNII: VH2XOU12IE) Product Characteristics Color red Score no score Shape ROUND Size 11mm Flavor Imprint Code E144 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42806-144-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 2 NDC: 42806-144-48 48 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 3 NDC: 42806-144-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065447 02/02/2015 Labeler - Epic Pharma, LLC (827915443) Registrant - Epic Pharma, LLC (827915443) Establishment Name Address ID/FEI Business Operations Epic Pharma, LLC 827915443 manufacture(42806-143, 42806-144)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.