Cherry Therastat by HART Health CHERRY THERASTAT
Cherry Therastat by
Drug Labeling and Warnings
Cherry Therastat by is a Otc medication manufactured, distributed, or labeled by HART Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
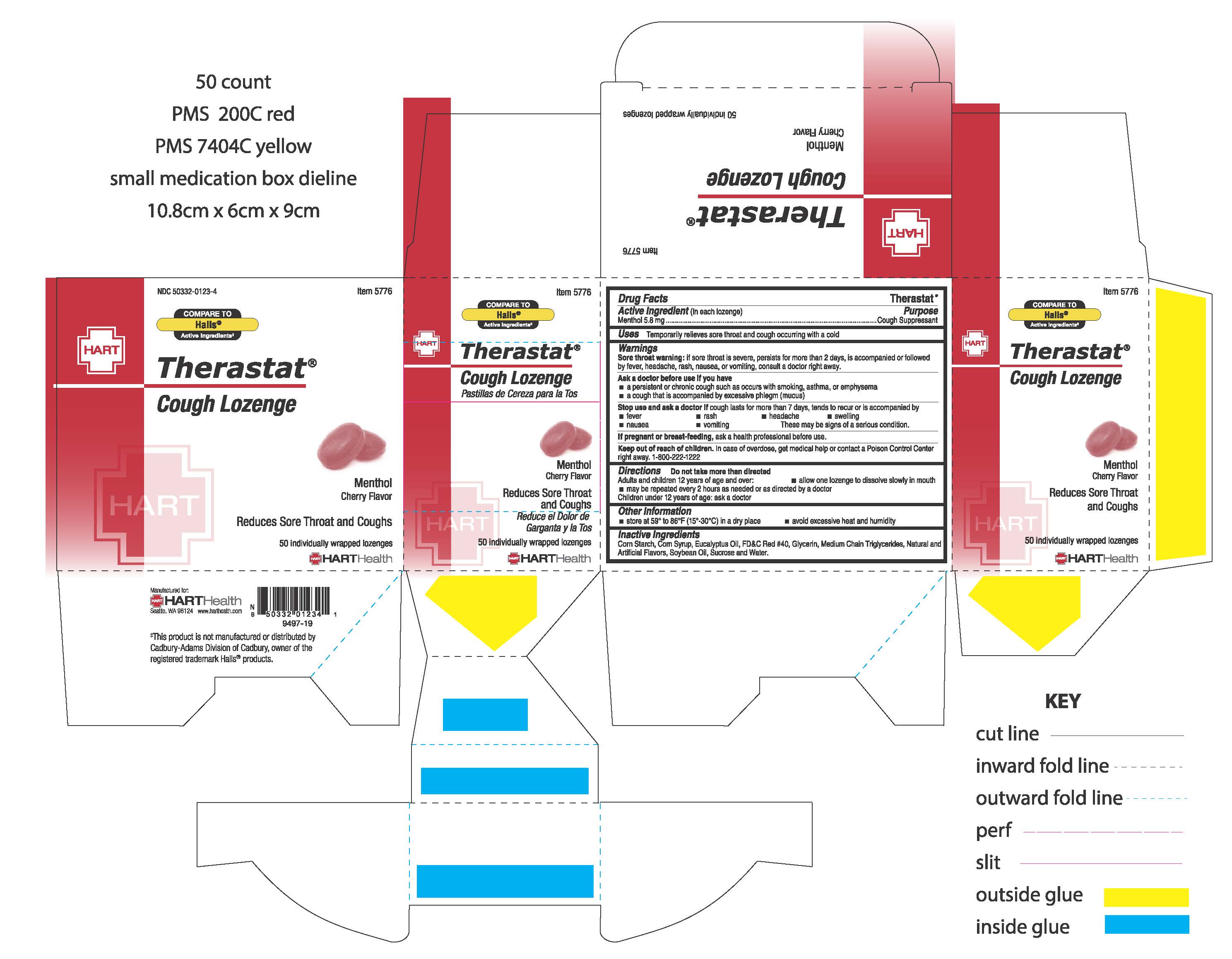
CHERRY THERASTAT- menthol lozenge
HART Health
----------
CHERRY THERASTAT
Warnings:
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by a fever, headache, rash, nausea, or vomiting, consult a doctor right away.
Ask a doctor before use if you have
- a persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- a cough that is accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if cough lasts for more than 7 days, tends to recur or is accompanied by
- fever
- rash
- headache
- swelling
- nausea
- vomiting
These may be signs of a serious condition
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. 1-800-222-1222
Directions:
Do not take more than directed
Adults and children 12 years of age and older:
- allow one lozenge to dissolve slowly in mouth
- may be repeated every 2 hours as needed or as directed by a doctor
Children under 12 years of age: ask a doctor
| CHERRY THERASTAT
menthol lozenge |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - HART Health (069560969) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.