SUPRAX- cefixime tablet, chewable
SUPRAX by
Drug Labeling and Warnings
SUPRAX by is a Prescription medication manufactured, distributed, or labeled by LUPIN PHARMA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Suprax (cefixime) chewable tablets and other antibacterial drugs, Suprax (cefixime) chewable tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
Suprax (cefixime) chewable tablet is a semisynthetic, cephalosporin antibiotic for oral administration. Chemically, it is (6R,7R)-7-[2-(2-Amino-4-thia-zolyl)glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0] oct-2-ene-2-carboxylic acid, 72-(Z)-[O-(carboxymethyl) oxime] trihydrate.
Molecular weight = 507.50 as the trihydrate. Chemical Formula is C16H15N5O7S2.3H2O
The structural formula for cefixime is:
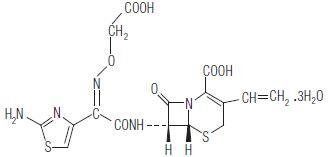
Each chewable tablet contains either 100 mg or 150 mg or 200 mg of cefixime as the trihydrate. In addition the tablet contains the following inactive ingredients: aspartame, colloidal silicon dioxide, crospovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, fantasy flavour permaseal, tutti frutti flavour and F D & C Red # 40 Aluminium Lake.
-
CLINICAL PHARMACOLOGY
Suprax (cefixime) chewable tablets are bioequivalent to oral suspension.
Suprax (cefixime) tablets, given orally, is about 40%-50% absorbed whether administered with or without food; however, time to maximal absorption is increased approximately 0.8 hours when administered with food. Suprax (cefixime) chewable tablets or oral suspension produces average peak concentrations approximately 25%-50% higher than the cefixime immediate-release tablets, when tested in normal adult volunteers. Two hundred and 400 mg doses of Suprax (cefixime) chewable tablets or oral suspension produce average peak concentrations of 3 mcg/mL (range 1 to 4.5 mcg/mL) and 4.6 mcg/mL (range 1.9 to 7.7 mcg/mL), respectively, when tested in normal adult volunteers. The area under the time versus concentration curve is greater by approximately 10%-25% with the Suprax (cefixime) chewable tablets or oral suspension than with the cefixime immediate-release tablets after doses of 100 to 400 mg, when tested in normal adult volunteers. This increased absorption should be taken into consideration if the Suprax (cefixime) chewable tablet or oral suspension is to be substituted for cefixime immediate-release tablet. Because of the lack of bioequivalence, cefixime immediate-release tablets should not be substituted for cefixime chewable tablets or oral suspension in the treatment of otitis media. (See DOSAGE AND ADMINISTRATION). Cross-over studies of cefixime immediate-release tablets versus chewable tablets or suspension have not been performed in children.
Peak serum concentrations occur between 2 and 6 hours following oral administration of 400 mg of Suprax (cefixime) chewable tablets or cefixime suspension.
Peak serum concentrations occur between 2 and 5 hours following a single administration of 200 mg of chewable tablets or suspension.
TABLE Serum Levels of Cefixime after Administration of Immediate-Release Tablets (mcg/mL)
DOSE
1h
2h
4h
6h
8h
12h
24h
100 mg 0.3 0.8 1 0.7 0.4 0.2 0.02 200 mg 0.7 1.4 2 1.5 1 0.4 0.03 400 mg 1.2 2.5 3.5 2.7 1.7 0.6 0.04 Serum Levels of Cefixime after Administration of Chewable Tablets or Oral Suspension (mcg/mL)
DOSE
1h
2h
4h
6h
8h
12h
24h
100 mg 0.7 1.1 1.3 0.9 0.6 0.2 0.02 200 mg 1.2 2.1 2.8 2 1.3 0.5 0.07 400 mg 1.8 3.3 4.4 3.3 2.2 0.8 0.07 Approximately 50% of the absorbed dose is excreted unchanged in the urine in 24 hours. In animal studies, it was noted that cefixime is also excreted in the bile in excess of 10% of the administered dose. Serum protein binding is concentration independent with a bound fraction of approximately 65%. In a multiple dose study conducted with a research formulation which is less bioavailable than the immediate release tablets or suspension, there was little accumulation of drug in serum or urine after dosing for 14 days. The serum half-life of cefixime in healthy subjects is independent of dosage form and averages 3-4 hours but may range up to 9 hours in some normal volunteers. Average AUCs at steady state in elderly patients are approximately 40% higher than average AUCs in other healthy adults.
In subjects with moderate impairment of renal function (20 to 40 mL/min creatinine clearance), the average serum half-life of cefixime is prolonged to 6.4 hours. In severe renal impairment (5 to 20 mL/min creatinine clearance), the half-life increased to an average of 11.5 hours. The drug is not cleared significantly from the blood by hemodialysis or peritoneal dialysis. However, a study indicated that with doses of 400 mg, patients undergoing hemodialysis have similar blood profiles as subjects with creatinine clearances of 21-60 mL/min. There is no evidence of metabolism of cefixime in vivo.
Adequate data on CSF levels of cefixime are not available.
Microbiology
As with other cephalosporins, bactericidal action of cefixime results from inhibition of cell-wall synthesis. Cefixime is highly stable in the presence of beta-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases, may be susceptible to cefixime. Cefixime has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE):
Gram-positive Organisms.
Streptococcus pneumoniae,
Streptococcus pyogenes.
Gram-negative Organisms.
Haemophilus influenzae
(beta-lactamase positive and negative strains),
Moraxella (Branhamella) catarrhalis
(most of which are beta-lactamase positive),
Escherichia coli,
Proteus mirabilis,
Neisseria gonorrhoeae
(including penicillinase- and non-penicillinase-producing strains).
Cefixime has been shown to be active in vitro against most strains of the following organisms; however, clinical efficacy has not been established.
Gram-positive Organisms.
Streptococcus agalactiae.
Gram-negative Organisms.
Haemophilus parainfluenzae
(beta-lactamase positive and negative strains),
Proteus vulgaris,
Klebsiella pneumoniae,
Klebsiella oxytoca,
Pasteurella multocida,
Providencia species,
Salmonella species,
Shigella species,
Citrobacter amalonaticus,
Citrobacter diversus,
Serratia marcescens.
Note: Pseudomonas species, strains of group D streptococci (including enterococci), Listeria monocytogenes, most strains of staphylococci (including methicillin-resistant strains) and most strains of Enterobacter are resistant to cefixime. In addition, most strains of Bacteroides fragilis and Clostridia are resistant to cefixime.
Susceptibility Tests:
Diffusion Techniques
Quantitative methods that require measurement of zone diameters give an estimate of antibiotic susceptibility. One such procedure1-3 has been recommended for use with disks to test susceptibility to cefixime. Interpretation involves correlation of the diameters obtained in the disk test with minimum inhibitory concentration (MIC) for cefixime.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 5-mcg cefixime disk should be interpreted according to the following criteria:
Recommended Susceptibility Ranges: Agar Disk Diffusion
Organisms
Resistant
Moderately Susceptible
Susceptible
Neisseria gonorrhoeaea
-- -- ≥31 mm All other organisms ≤15 mm 16 - 18 mm ≥ 19 mm a Using GC Agar Base with a defined 1% supplement without cysteine. A report of "Susceptible" indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of "Moderately Susceptible" indicates that inhibitory concentrations of the antibiotic may well be achieved if high dosage is used or if the infection is confined to tissues and fluids (e.g. urine) in which high antibiotic levels are attained. A report of "Resistant" indicates that achievable concentrations of the antibiotic are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 5-mcg disk should give the following zone diameter:
Header$Organism
Zone diameter (mm)
- * Using GC Agar Base with a defined 1% supplement without cysteine.
E. coli ATCC 25922
23-27
N. gonorrhoeae ATCC 49226*
37-45
The class disk for cephalosporin susceptibility testing (the cephalothin disk) is not appropriate because of spectrum differences with cefixime. The 5-mcg cefixime disk should be used for all in vitro testing of isolates.
Dilution Techniques
Broth or agar dilution methods can be used to determine the minimum inhibitory concentration (MIC) value for susceptibility of bacterial isolates to cefixime. The recommended susceptibility breakpoints are as follows:
MIC Interpretive Standards (mcg/mL)
Organisms
Resistant
Moderately Susceptible
Susceptible
Neisseria gonorrhoeaea
-- -- ≤ 0.25 All other organisms ≥ 4 2 ≤ 1 As with standard diffusion methods, dilution procedures require the use of laboratory control organisms. Standard cefixime powder should give the following MIC ranges in daily testing of quality control organisms:
Organism
MIC range (mcg/mL)
aUsing GC Agar Base with a defined 1% supplement without cysteine.
E. coli ATCC 25922
0.25-1
S. aureus ATCC 29213
8-32
N. gonorrhoeae ATCC 49226a
0.008-0.03
-
INDICATIONS AND USAGE
To reduce the development of drug resistant bacteria and maintain the effectiveness of Suprax (cefixime) chewable tablets and other antibacterial drugs, Suprax (cefixime) chewable tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antimicrobial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Suprax (cefixime) chewable tablets are indicated in the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
Uncomplicated Urinary Tract Infections caused by Escherichia coli and Proteus mirabilis.
Otitis Media caused by Haemophilus influenzae (beta-lactamase positive and negative strains), Moraxella (Branhamella) catarrhalis, (most of which are beta-lactamase positive) and S. pyogenes*.
Note: For information on otitis media caused by Streptococcus pneumoniae, see CLINICAL STUDIES section.
Pharyngitis and Tonsillitis, caused by S. pyogenes.
Note: Penicillin is the usual drug of choice in the treatment of S. pyogenes infections, including the prophylaxis of rheumatic fever. Suprax (cefixime) chewable tablets are generally effective in the eradication of S. pyogenes from the nasopharynx; however, data establishing the efficacy of Suprax (cefixime) chewable tablets in the subsequent prevention of rheumatic fever are not available.
Acute Bronchitis and Acute Exacerbations of Chronic Bronchitis, caused by Streptococcus pneumoniae and Haemophilus influenzae (beta-lactamase positive and negative strains).
Uncomplicated gonorrhea (cervical/urethral), caused by Neisseria gonorrhoeae (penicillinase-and non-penicillinase- producing strains).
Appropriate cultures and susceptibility studies should be performed to determine the causative organism and its susceptibility to cefixime; however, therapy may be started while awaiting the results of these studies. Therapy should be adjusted, if necessary, once these results are known.
* Efficacy for this organism in this organ system was studied in fewer than 10 infections.
-
CLINICAL STUDIES
In clinical trials of otitis media in nearly 400 children between the ages of 6 months to 10 years, Streptococcus pneumoniae was isolated from 47% of the patients, Haemophilus influenzae from 34%, Moraxella (Branhamella) catarrhalis from 15% and S. pyogenes from 4%.
The overall response rate of Streptococcus pneumoniae to cefixime was approximately 10% lower and that of Haemophilus influenzae or Moraxella (Branhamella) catarrhalis approximately 7% higher (12% when beta-lactamase positive strains of H. influenzae are included) than the response rates of these organisms to the active control drugs.
In these studies, patients were randomized and treated with either cefixime at dose regimens of 4 mg/kg BID or 8 mg/kg QD, or with a standard antibiotic regimen. Sixty-nine to 70% of the patients in each group had resolution of signs and symptoms of otitis media when evaluated 2 to 4 weeks post-treatment, but persistent effusion was found in 15% of the patients. When evaluated at the completion of therapy, 17% of patients receiving cefixime and 14% of patients receiving effective comparative drugs (18% including those patients who had Haemophilus influenzae resistant to the control drug and who received the control antibiotic) were considered to be treatment failures. By the 2 to 4 week follow-up, a total of 30%-31% of patients had evidence of either treatment failure or recurrent disease.
Bacteriological Outcome of Otitis Media at Two to Four Weeks Post-Therapy Based on Repeat Middle Ear Fluid Culture or Extrapolation from Clinical Outcome
(a) Number eradicated/number isolated.
(b) An additional 20 beta-lactamase positive strains of Haemophilus influenzae were isolated, but were excluded from this analysis because they were resistant to the control antibiotic. In nineteen of these, the clinical course could be assessed and a favorable outcome occurred in 10. When these cases are included in the overall bacteriological evaluation of therapy with the control drugs, 140/185 (76%) of pathogens were considered to be eradicated.
Organism
Cefixime(a)
4 mg/kg BID
Cefixime(a)
8 mg/kg QD
Control(a)
drugs
Streptococcus pneumoniae
48/70 (69%)
18/22 (82%)
82/100 (82%)
Haemophilus influenzae
beta-lactamase negative
24/34 (71%)
13/17 (76%)
23/34 (68%)
Haemophilus influenzae
beta-lactamase positive
17/22 (77%)
9/12 (75%)
1/1 (b)
Moraxella (Branhamella)
catarrhalis
26/31 (84%)
5/5
18/24 (75%)
S. pyogenes
5/5
3/3
6/7
All Isolates
120/162 (74%)
48/59 (81%)
130/166 (78%)
- CONTRAINDICATIONS
-
WARNINGS
BEFORE THERAPY WITH SUPRAX (CEFIXIME) CHEWABLE TABLET IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO SUPRAX (CEFIXIME) CHEWABLE TABLETS OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Anaphylactic/anaphylactoid reactions (including shock and fatalities) have been reported with the use of cefixime.
Antibiotics, including Suprax (cefixime) chewable tablets, should be administered cautiously to any patient who has demonstrated some form of allergy, particularly to drugs.
Treatment with broad spectrum antibiotics, including Suprax (cefixime) chewable tablets, alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of severe antibiotic-associated diarrhea including pseudomembranous colitis.
Pseudomembranous colitis has been reported with the use of Suprax (cefixime) chewable tablets and other broad-spectrum antibiotics (including macrolides, semisynthetic penicillins, and cephalosporins); therefore, it is important to consider this diagnosis in patients who develop diarrhea in association with the use of antibiotics. Symptoms of pseudomembranous colitis may occur during or after antibiotic treatment and may range in severity from mild to life-threatening. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, management should include fluids, electrolytes, and protein supplementation. If the colitis does not improve after the drug has been discontinued, or if the symptoms are severe, oral vancomycin is the drug of choice for antibiotic-associated pseudomembranous colitis produced by C. difficile. Other causes of colitis should be excluded.
-
PRECAUTIONS
General
Prescribing Suprax (cefixime) chewable tablets in the absence of a proven or strongly suspected bacterial infection of a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
The possibility of the emergence of resistant organisms which might result in overgrowth should be kept in mind, particularly during prolonged treatment. In such use, careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
The dose of Suprax (cefixime) chewable tablets should be adjusted in patients with renal impairment as well as those undergoing continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis (HD). Patients on dialysis should be monitored carefully. (See DOSAGE AND ADMINISTRATION.)
Suprax (cefixime) chewable tablets should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
Drug Interactions
Carbamazepine: Elevated carbamazepine levels have been reported in postmarketing experience when cefixime is administered concomitantly. Drug monitoring may be of assistance in detecting alterations in carbamazepine plasma concentrations.
Warfarin and Anticoagulants: Increased prothrombin time, with or without clinical bleeding, has been reported when cefixime is administered concomitantly.
Drug/Laboratory Test Interactions
A false-positive reaction for ketones in the urine may occur with tests using nitroprusside but not with those using nitroferricyanide.
The administration of cefixime may result in a false-positive reaction for glucose in the urine using Clinitest®**, Benedict’s solution, or Fehling’s solution. It is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix®** or TesTape®**) be used.
A false-positive direct Coombs test has been reported during treatment with other cephalosporin antibiotics; therefore, it should be recognized that a positive Coombs test may be due to the drug.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime studies in animals to evaluate carcinogenic potential have not been conducted. Cefixime did not cause point mutations in bacteria or mammalian cells, DNA damage, or chromosome damage in vitro and did not exhibit clastogenic potential in vivo in the mouse micronucleus test. In rats, fertility and reproductive performance were not affected by cefixime at doses up to 125 times the adult therapeutic dose.
Usage in Pregnancy
Pregnancy Category B. Reproduction studies have been performed in mice and rats at doses up to 400 times the human dose and have revealed no evidence of harm to the fetus due to cefixime. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Cefixime has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
Nursing Mothers
It is not known whether cefixime is excreted in human milk. Consideration should be given to discontinuing nursing temporarily during treatment with this drug.
Pediatric Use
Safety and effectiveness of cefixime in children aged less than six months old have not been established.
The incidence of gastrointestinal adverse reactions, including diarrhea and loose stools, in the pediatric patients receiving the suspension, was comparable to the incidence seen in adult patients receiving immediate-release tablets.
Information for Patients
Patients should be counseled that antibacterial drugs, including Suprax (cefixime) chewable tablets, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Suprax (cefixime) chewable tablets is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Suprax (cefixime) chewable tablets or other antibacterial drugs in the future.
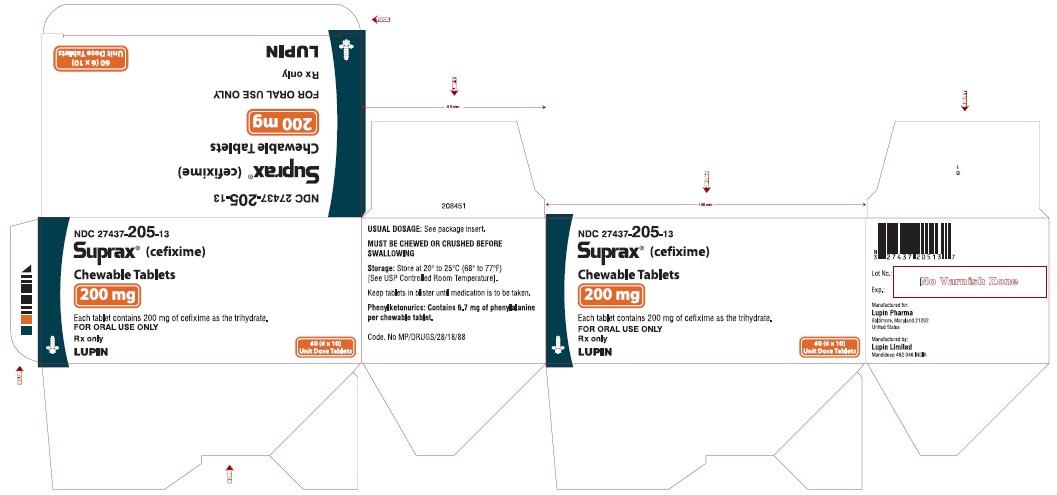
Phenylketonurics: Contains 3.3 mg, 5 mg and 6.7 mg of phenylalanine per 100 mg, 150 mg and 200 mg tablet respectively.
-
ADVERSE REACTIONS
Most of adverse reactions observed in clinical trials were of a mild and transient nature. Five percent (5%) of patients in the U.S. trials discontinued therapy because of drug-related adverse reactions. The most commonly seen adverse reactions in U.S. trials of the immediate-release tablet formulation were gastrointestinal events, which were reported in 30% of adult patients on either the BID or the QD regimen. Clinically mild gastrointestinal side effects occurred in 20% of all patients, moderate events occurred in 9% of all patients and severe adverse reactions occurred in 2% of all patients. Individual event rates included diarrhea 16%, loose or frequent stools 6%, abdominal pain 3%, nausea 7%, dyspepsia 3%, and flatulence 4%. The incidence of gastrointestinal adverse reactions, including diarrhea and loose stools, in pediatric patients receiving the suspension was comparable to the incidence seen in adult patients receiving immediate-release tablets.
These symptoms usually responded to symptomatic therapy or ceased when cefixime was discontinued.
Several patients developed severe diarrhea and/or documented pseudomembranous colitis, and a few required hospitalization.
The following adverse reactions have been reported following the use of cefixime. Incidence rates were less than 1 in 50 (less than 2%), except as noted above for gastrointestinal events.
Gastrointestinal (see above): Diarrhea, loose stools, abdominal pain, dyspepsia, nausea, and vomiting. Several cases of documented pseudomembranous colitis were identified during the studies. The onset of pseudomembranous colitis symptoms may occur during or after therapy.
Hypersensitivity Reactions: Anaphylactic/anaphylactoid reactions (including shock and fatalities), skin rashes, urticaria, drug fever, pruritus, angioedema, and facial edema. Erythema multiforme, Stevens-Johnson syndrome, and serum sickness-like reactions have been reported.
Hepatic: Transient elevations in SGPT, SGOT, alkaline phosphatase, hepatitis, jaundice.
Renal: Transient elevations in BUN or creatinine, acute renal failure.
Central Nervous System: Headaches, dizziness, seizures.
Hemic and Lymphatic Systems: Transient thrombocytopenia, leukopenia, neutropenia, and eosinophilia. Prolongation in prothrombin time was seen rarely.
Abnormal Laboratory Tests: Hyperbilirubinemia.
Other: Genital pruritus, vaginitis, candidiasis, toxic epidermal necrolysis.
In addition to the adverse reactions listed above which have been observed in patients treated with cefixime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Adverse reactions: Allergic reactions, superinfection, renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, and colitis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. (See DOSAGE AND ADMINISTRATIONand OVERDOSAGE.) If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Abnormal Laboratory Tests: Positive direct Coombs test, elevated LDH, pancytopenia, agranulocytosis.
-
OVERDOSAGE
Gastric lavage may be indicated; otherwise, no specific antidote exists. Cefixime is not removed in significant quantities from the circulation by hemodialysis or peritoneal dialysis. Adverse reactions in small numbers of healthy adult volunteers receiving single doses up to 2 g of cefixime did not differ from the profile seen in patients treated at the recommended doses.
-
DOSAGE AND ADMINISTRATION
ALL RECOMMENDED DOSAGE FOR CEFIXIME SUSPENSION ARE INCLUDED IN THIS SECTION FOR INFORMATIONAL PURPOSE ONLY. SUPRAX (CEFIXIME) CHEWABLE TABLETS 100 MG ARE APPROPRIATE FOR A 100 MG DOSE, SUPRAX (CEFIXIME) CHEWABLE TABLETS 150 MG ARE APPROPRIATE FOR A 150 MG DOSE AND SUPRAX (CEFIXIME) CHEWABLE TABLETS 200 MG ARE APPROPRIATE FOR A 200 MG DOSE.
SUPRAX (CEFIXIME) CHEWABLE TABLETS MUST BE CHEWED OR CRUSHED BEFORE SWALLOWING.
Adults: The recommended dose is 400 mg daily. For the treatment of uncomplicated cervical/urethral gonococcal infections, a single oral dose of 400 mg is recommended.
Children: The recommended dose is 8 mg/kg/day of the cefixime. This may be administered as a single daily dose or may be given in two divided doses, as 4 mg/kg every 12 hours.
PEDIATRIC DOSAGE CHART
100 mg/5 mL
200 mg/5 mL
Suprax (cefixime) Chewable Tablet
Patient Weight
(kg)
Dose/Day
mg
Dose/Day
mL
Dose/Day
tsp of Suspension
Dose/Day
mL
Dose/Day
tsp of Suspension
Dose
6.25
50
2.5
½
1.25
¼
---
12.5
100
5
1
2.5
½
1 tablet of 100 mg
18.75
150
7.5
1½
3.75
¾
1 tablet of 150 mg
25
200
10
2
5
1
1 tablet of 200 mg
31.25
250
12.5
2½
6.25
1¼
1 tablet of 100 mg and 1 tablet of 150 mg
37.5
300
15
3
7.5
1½
2 tablets of 150 mg
Children weighing more than 50 kg or older than 12 years should be treated with the recommended adult dose.
Otitis media should be treated with Suprax (cefixime) chewable tablets or suspension. Clinical studies of otitis media were conducted with Suprax (cefixime) chewable tablets or suspension, and the Suprax (cefixime) chewable tablets or suspension results in higher peak blood levels than the immediate-release tablets when administered at the same dose. Therefore, the immediate-release tablets should not be substituted for Suprax (cefixime) chewable tablets or suspension in the treatment of otitis media. (See CLINICAL PHARMACOLOGY.)
Efficacy and safety in infants aged less than six months have not been established.
In the treatment of infections due to S. pyogenes, a therapeutic dosage of Suprax (cefixime) chewable tablets should be administered for at least 10 days.
Renal Impairment
Suprax (cefixime) chewable tablets may be administered in the presence of impaired renal function. Normal dose and schedule may be employed in patients with creatinine clearances of 60 mL/min or greater. Patients whose clearance is between 21 and 60 mL/min or patients who are on renal hemodialysis may be given 75% of the standard dosage at the standard dosing interval (i.e., 300 mg daily). Patients whose clearance is < 20 mL/min, or patients who are on continuous ambulatory peritoneal dialysis may be given half the standard dosage at the standard dosing interval (i.e., 200 mg daily). Neither hemodialysis nor peritoneal dialysis remove significant amounts of drug from the body.
-
HOW SUPPLIED
Suprax (cefixime) chewable tablets, 100 mg, 150 mg and 200 mg
100 mg tablet - Pink round tablet debossed "SUPRAX 100" on one side and "LUPIN" on other side.
NDC: 27437-203-13 - Outer carton containing 6 monocartons (NDC: 27437-203-11) each containing 10’s blister.
NDC: 27437-203-10 - Bottle of 10’s
NDC: 27437-203-08 - Bottle of 50’s
150 mg tablet - Pink round tablet debossed "SUPRAX 150" on one side and "LUPIN" on other side.
NDC: 27437-204-13 - Outer carton containing 6 monocartons (NDC: 27437-204-11) each containing 10’s blister.
NDC: 27437-204-10 - Bottle of 10’s
NDC: 27437-204-08 - Bottle of 50’s
200 mg tablet - Pink round tablet debossed "SUPRAX 200" on one side and "LUPIN" on other side.
NDC: 27437-205-13 - Outer carton containing 6 monocartons (NDC: 27437-205-11) each containing 10’s blister.
NDC: 27437-205-10 - Bottle of 10’s
NDC: 27437-205-08 - Bottle of 50’s
Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
-
REFERENCES
- Bauer AW, Kirby WMM, Sherris JC, et al.: Antibiotic susceptibility testing by a standard single disk method. Am J Clin Pathol 1966; 45:493.
- National Committee for Clinical Laboratory Standards, Approved Standard: Performance Standards for Antimicrobial Disk Susceptibility Tests (M2-A3), December 1984.
- Standardized disk susceptibility test. Federal Register 1974; 39 (May 30): 19182-19184.
-
SPL UNCLASSIFIED SECTION
**Clinitest® and Clinistix® are registered trademarks of Ames Division, Miles Laboratories, Inc. Tes-Tape® is a registered trademark of Eli Lilly and Company.
Manufactured for:
Lupin Pharma
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Mandideep 462 026
INDIA
October 2012 ID #216547
-
PRINCIPAL DISPLAY PANEL
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
100 mg
NDC: 27437-203-10
BOTTLE LABEL
10 TABLETS

SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
100 mg
NDC: 27437-203-11
BLISTER FOIL LABEL
10 TABLETS SINGLE UNIT PACKAGE
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
100 mg
NDC: 27437-203-11
CARTON LABEL
10 TABLETS SINGLE UNIT PACKAGE

SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
100 mg
NDC: 27437-203-13
CARTON LABEL
6 x 10 TABLETS UNIT DOSE
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
150 mg
NDC: 27437-204-10
BOTTLE LABEL
10 TABLETS

SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
150 mg
NDC: 27437-204-11
BLISTER FOIL LABEL
10 TABLETS SINGLE UNIT PACKAGE
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
150 mg
NDC: 27437-204-11
CARTON LABEL
10 TABLETS SINGLE UNIT PACKAGE
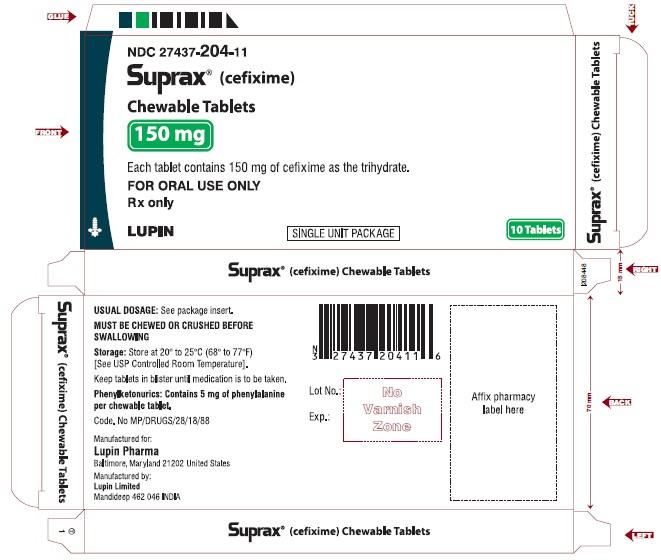
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
150 mg
NDC: 27437-204-13
CARTON LABEL
6 x 10 TABLETS UNIT DOSE
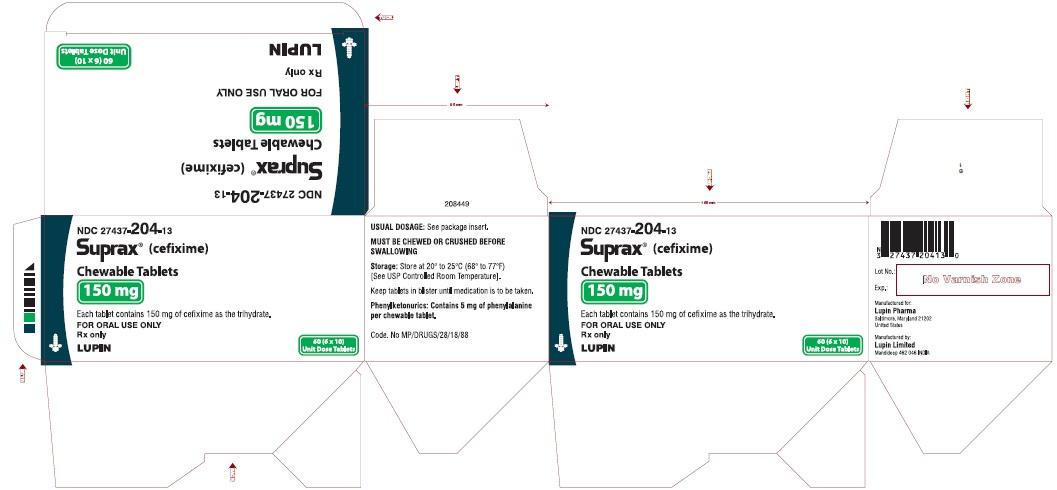
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
200 mg
NDC: 27437-205-10
BOTTLE LABEL
10 TABLETS

SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
200 mg
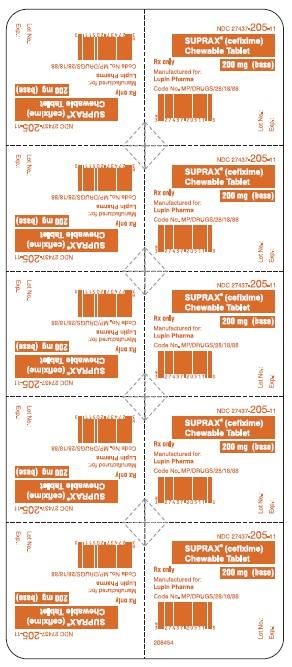
NDC: 27437-205-11
BLISTER FOIL LABEL
10 TABLETS SINGLE UNIT PACKAGE
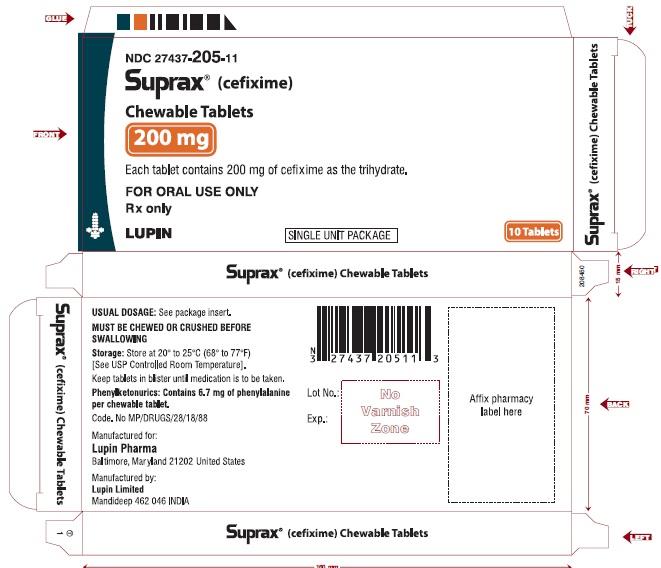
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
200 mg
NDC: 27437-205-11
CARTON LABEL
10 TABLETS SINGLE UNIT PACKAGE
SUPRAX® (CEFIXIME) CHEWABLE TABLETS
Rx Only
200 mg
NDC: 27437-205-13
CARTON LABEL
6 x 10 TABLETS UNIT DOSE
-
INGREDIENTS AND APPEARANCE
SUPRAX
cefixime tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 27437-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFIXIME (UNII: 97I1C92E55) (CEFIXIME ANHYDROUS - UNII:XZ7BG04GJX) CEFIXIME ANHYDROUS 100 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (UNII: 68401960MK) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color PINK (Pink) Score no score Shape ROUND (Round) Size 11mm Flavor TUTTI FRUTTI Imprint Code SUPRAX100;LUPIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 27437-203-13 6 in 1 CARTON 1 NDC: 27437-203-11 1 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC: 27437-203-10 10 in 1 BOTTLE 3 NDC: 27437-203-08 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065380 10/11/2012 SUPRAX
cefixime tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 27437-205 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFIXIME (UNII: 97I1C92E55) (CEFIXIME ANHYDROUS - UNII:XZ7BG04GJX) CEFIXIME ANHYDROUS 200 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (UNII: 68401960MK) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color PINK (Pink) Score no score Shape ROUND (Round) Size 14mm Flavor TUTTI FRUTTI Imprint Code SUPRAX200;LUPIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 27437-205-13 6 in 1 CARTON 1 NDC: 27437-205-11 1 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC: 27437-205-10 10 in 1 BOTTLE 3 NDC: 27437-205-08 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065380 10/11/2012 Labeler - LUPIN PHARMA (965791259)
Trademark Results [SUPRAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUPRAX 86164468 4660961 Live/Registered |
Waterbrands, LLC 2014-01-13 |
 SUPRAX 73707543 1500158 Dead/Cancelled |
AMERICAN CYANAMID COMPANY 1988-01-25 |
 SUPRAX 73604097 1456050 Live/Registered |
AMERICAN CYANAMID COMPANY 1986-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.