DEXTROAMPHETAMINE SULFATE EXTENDED RELEASE CAPSULES CII RX ONLY
Dextroamphetamine Sulfate by
Drug Labeling and Warnings
Dextroamphetamine Sulfate by is a Prescription medication manufactured, distributed, or labeled by Lineage Therapeutics Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROAMPHETAMINE SULFATE EXTENDED-RELEASE- dextroamphetamine sulfate capsule, extended release
Lineage Therapeutics Inc
----------
DEXTROAMPHETAMINE SULFATE EXTENDED RELEASE CAPSULES CII
RX ONLY
WARNING:
| AMPHETAMINES HAVE A HIGH POTENTIAL
FOR ABUSE. ADMINISTRATION OF AMPHETAMINES FOR PROLONGED
PERIODS OF TIME MAY LEAD TO DRUG DEPENDENCE AND MUST BE
AVOIDED. PARTICULAR ATTENTION SHOULD BE PAID
TO THE POSSIBILITY OF SUBJECTS OBTAINING AMPHETAMINES
FOR NON-THERAPEUTIC USE OR DISTRIBUTION TO OTHERS, AND
THE DRUGS SHOULD BE PRESCRIBED OR DISPENSED
SPARINGLY. MISUSE OF AMPHETAMINES MAY CAUSE SUDDEN DEATH AND SERIOUS CARDIOVASCULAR ADVERSE EVENTS. |
DESCRIPTION:
Dextroamphetamine sulfate is the dextro isomer of the compound d,l-amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is d-alpha-methylphenethylamine, and is present in all forms of dextroamphetamine sulfate as the neutral sulfate.
Structural formula:
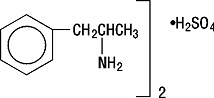
Each extended-release capsule is so prepared that an initial dose is released promptly and the remaining medication is released gradually over a prolonged period. Each small spherical capsule, contains dextroamphetamine sulfate. The 5 mg capsule is imprinted “327” on the YELLOW cap, and “CP” and “5 mg” on the body. The 10 mg capsule is imprinted “328” on the ORANGE cap, and “CP” and “10 mg” on the body. The 15 mg capsule is imprinted “329” on the RED cap, and “CP” and “15 mg” on the body. Product reformulation in 1996 has caused a minor change in the color of the time-released pellets within each capsule. Inactive ingredients now consist of cetyl alcohol, D&C Yellow No. 10, dibutyl sebacate, ethylcellulose, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, hypromellose, polyethylene glycol, povidone, sodium lauryl sulfate, sugar spheres, and trace amounts of other inactive ingredients.
CLINICAL PHARMACOLOGY:
Amphetamines are noncatecholamine, sympathomimetic amines with CNS stimulant activity. Peripheral actions include elevations of systolic and diastolic blood pressures and weak bronchodilator and respiratory stimulant action.
There is neither specific evidence that clearly establishes the mechanism whereby amphetamines produce mental and behavioral effects in children, nor conclusive evidence regarding how these effects relate to the condition of the central nervous system.
Dextroamphetamine sulfate extended release capsules are formulated to release the active drug substance in vivo in a more gradual fashion than the standard formulation, as demonstrated by blood levels. The formulation has not been shown superior in effectiveness over the same dosage of the standard, noncontrolled-release formulations given in divided doses.
Pharmacokinetics:
The pharmacokinetics of the tablet and extended-release capsule were compared in 12 healthy subjects. The extent of bioavailability of the extended-release capsule was similar compared to the immediate-release tablet. Following administration of three 5-mg tablets, average maximal dextroamphetamine plasma concentrations (Cmax) of 36.6 ng/mL were achieved at approximately 3 hours. Following administration of one 15-mg extended-release capsule, maximal dextroamphetamine plasma concentrations were obtained approximately 8 hours after dosing. The average Cmax was 23.5 ng/mL. The average plasma T½ was similar for both the tablet and extended-release capsule and was approximately 12 hours.
In 12 healthy subjects, the rate and extent of dextroamphetamine absorption were similar following administration of the extended-release capsule formulation in the fed (58 to 75 gm fat) and fasted state.
INDICATIONS AND USAGE:
Dextroamphetamine sulfate is indicated in:
Attention Deficit Disorder with Hyperactivity
As an integral part of a total treatment program that typically includes other measures (psychological, educational, social) for patients (ages 6 years to 16 years) with this syndrome. A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in 2 or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least 6 of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoid tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least 6 of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go”; excessive talking; blurting answers; can't wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.
Special Diagnostic Considerations
Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use of medical and special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the patient and not solely on the presences of the required number of DSM-IV characteristics.
Need for Comprehensive Treatment Program
Dextroamphetamine sulfate is indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Stimulants are not intended for use in patients who exhibit symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms.
CONTRAINDICATIONS
Advanced arteriosclerosis, symptomatic cardiovascular disease,
moderate to severe hypertension, hyperthyroidism, known hypersensitivity
or idiosyncrasy to the sympathomimetic amines,
glaucoma.
Agitated
states.
Patients with a
history of drug
abuse.
Known hypersensitivity or idiosyncrasy to amphetamine.
In patients known to be hypersensitive to amphetamine, or other components of dextroamphetamine sulfate. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other amphetamine products [see Adverse Reactions]
Patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of stopping MAOIs (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis [see Warnings and Drug Interactions].
WARNINGS
Sudden Death in Patients With Pre-existing Structural Cardiac Abnormalities or Other Serious Heart Problems
Children and Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug.
Adults
Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs (see CONTRAINDICATIONS).
Hypertension and Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2 to 4 mmHg) and average heart rate (about 3 to 6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia (see CONTRAINDICATIONS).
Assessing Cardiovascular Status in Patients Being Treated With Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Pre-Existing Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3,482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of, or worsening of, aggressive behavior or hostility.
Long-Term Suppression of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children older than 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Peripheral Vasculopathy, including Raynaud's phenomenon
Stimulants, including dextroamphetamine sulfate, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John’s Wort [see Drug Interactions)]. Amphetamines and amphetamine derivatives are known to be metabolized, to some degree, by cytochrome P450 2D6 (CYP2D6) and display minor inhibition of CYP2D6 metabolism [see Clinical Pharmacology]. The potential for a pharmacokinetic interaction exists with the co-administration of CYP2D6 inhibitors which may increase the risk with increased exposure to dextroamphetamine sulfate. In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 [see Drug Interactions].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of dextroamphetamine sulfate with MAOI drugs is contraindicated [see Contraindications].
Discontinue treatment with dextroamphetamine sulfate and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of dextroamphetamine sulfate with other serotonergic drugs or CYP2D6 inhibitors is clinically warranted, initiate dextroamphetamine sulfate with lower doses, monitor patients for the emergence of serotonin syndrome during drug initiation or titration, and inform patients of the increased risk for serotonin syndrome.
PRECAUTIONS
General
The least amount feasible should be prescribed or dispensed at 1 time in order to minimize the possibility of overdosage.
Information for Patients:
Amphetamines may impair the ability of the patient to
engage in potentially hazardous activities such as operating
machinery or vehicles; the patient should therefore be cautioned
accordingly.
Prescribers or other health professionals
should inform patients, their families, and their caregivers
about the benefits and risks associated with treatment with
dextroamphetamine and should counsel them in its appropriate
use. A patient Medication Guide is available for
Dextroamphetamine Sulfate Extended Release Capsules. The
prescriber or health professional should instruct patients,
their families, and their caregivers to read the Medication
Guide and should assist them in understanding its contents.
Patients should be given the opportunity to discuss the contents
of the Medication Guide and to obtain answers to any questions
they may have. The complete text of the Medication Guide is
reprinted at the end of this document.
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]
- Instruct patients beginning treatment with dextroamphetamine sulfate about the risk of peripheral vasculopathy, including Raynaud's phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking dextroamphetamine sulfate.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Drug Interactions
Acidifying Agents
Lower blood levels and efficacy of amphetamines. Increase dose based on clinical response. Examples of acidifying agents include gastrointestinal acidifying agents (e.g., guanethidine, reserpine, glutamic acid HCl, ascorbic acid) and urinary acidifying agents (e.g., ammonium chloride, sodium acid phosphate, methenamine salts).
Alkalinizing Agents
Increase blood levels and potentiate the action of amphetamine. Co-administration of dextroamphetamine sulfate and gastrointestinal alkalinizing agents should be avoided. Examples of alkalinizing agents include gastrointestinal alkalinizing agents (e.g., sodium bicarbonate) and urinary alkalinizing agents (e.g. acetazolamide, some thiazides).
Tricyclic Antidepressants
May enhance the activity of tricyclic or sympathomimetic agents causing striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated. Monitor frequently and adjust or use alternative therapy based on clinical response. Examples of tricyclic antidepressants include desipramine, Protriptyline.
CYP2D6 Inhibitors
The concomitant use of dextroamphetamine sulfate and CYP2D6 inhibitors may increase the exposure of dextroamphetamine sulfate compared to the use of the drug alone and increase the risk of serotonin syndrome. Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome particularly during dextroamphetamine sulfate initiation and after a dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine sulfate and the CYP2D6 inhibitor [see Warnings, Overdosage]. Examples of CYP2D6 Inhibitors include paroxetine and fluoxetine (also serotonergic drugs), quinidine, ritonavir.
Serotonergic Drugs
The concomitant use of dextroamphetamine sulfate and serotonergic drugs increases the risk of serotonin syndrome. Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during dextroamphetamine sulfate initiation or dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine sulfate and the concomitant serotonergic drug(s) [see Warnings and Precautions]. Examples of serotonergic drugs include selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John’s Wort.
MAO Inhibitors
Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure. Do not administer dextroamphetamine sulfate concomitantly or within 14 days after discontinuing MAOI [see Contraindications and Warnings]. Examples of MAOIs include selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue.
Proton Pump Inhibitors
Time to maximum concentration (Tmax) of amphetamine is decreased compared to when administered alone. Monitor patients for changes in clinical effect and adjust therapy based on clinical response. An example of a proton pump inhibitor is omeprazole.
Chlorpromazine
Chlorpromazine blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines, and can be used to treat amphetamine poisoning.
Haloperidol
Haloperidol blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamines.
Methenamine Therapy
Urinary excretion of amphetamines is increased, and efficacy is reduced, by acidifying agents used in methenamine therapy.
Phenobarbital
Amphetamines may delay intestinal absorption of phenobarbital; co-administration of phenobarbital may produce a synergistic anticonvulsant action.
Phenytoin
Amphetamines may delay intestinal absorption of phenytoin; co-administration of phenytoin may produce a synergistic anticonvulsant action.
Drug/Laboratory Test Interactions
Amphetamines can cause a significant elevation in plasma corticosteroid levels. This increase is greatest in the evening.
Amphetamines may interfere with urinary steroid determinations.
Carcinogenesis/Mutagenesis
Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of dextroamphetamine sulfate have not been performed.
Pregnancy
Teratogenic Effects
Pregnancy Category C. Dextroamphetamine sulfate has been shown to have embryotoxic and teratogenic effects when administered to A/Jax mice and C57BL mice in doses approximately 41 times the maximum human dose. Embryotoxic effects were not seen in New Zealand white rabbits given the drug in doses 7 times the human dose nor in rats given 12.5 times the maximum human dose. While there are no adequate and well-controlled studies in pregnant women, there has been 1 report of severe congenital bony deformity, tracheoesophageal fistula, and anal atresia (VATER association) in a baby born to a woman who took dextroamphetamine sulfate with lovastatin during the first trimester of pregnancy. Dextroamphetamine sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use
Long-term effects of amphetamines in pediatric patients have not been well established.
Dextroamphetamine sulfate is not recommended for use in pediatric patients younger than 6 years of age with Attention Deficit Disorder with Hyperactivity described under INDICATIONS AND USAGE.
Clinical experience suggests that in psychotic children, administration of amphetamines may exacerbate symptoms of behavior disturbance and thought disorder.
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette’s syndrome. Therefore, clinical evaluation for tics and Tourette’s syndrome in children and their families should precede use of stimulant medications.
Data are inadequate to determine whether chronic administration of amphetamines may be associated with growth inhibition; therefore, growth should be monitored during treatment.
Drug treatment is not indicated in all cases of Attention Deficit Disorder with Hyperactivity and should be considered only in light of the complete history and evaluation of the child. The decision to prescribe amphetamines should depend on the physician’s assessment of the chronicity and severity of the child’s symptoms and their appropriateness for his or her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with amphetamines is usually not indicated.
ADVERSE REACTIONS
Cardiovascular
Palpitations, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System
Psychotic episodes at recommended doses (rare), overstimulation, restlessness, dizziness, insomnia, euphoria, dyskinesia, dysphoria, tremor, headache, exacerbation of motor and phonic tics, and Tourette’s syndrome.
DRUG ABUSE AND DEPENDENCE
Dextroamphetamine sulfate is a Schedule II controlled
substance.
Amphetamines have been extensively abused. Tolerance,
extreme psychological dependence and severe social disability have
occurred. There are reports of patients who have increased the dosage to
many times that recommended. Abrupt cessation following prolonged high
dosage administration results in extreme fatigue and mental depression;
changes are also noted on the sleep EEG.
Manifestations of chronic
intoxication with amphetamines include severe dermatoses, marked
insomnia, irritability, hyperactivity, and personality changes. The most
severe manifestation of chronic intoxication is psychosis, often
clinically indistinguishable from schizophrenia. This is rare with oral
amphetamines.
OVERDOSAGE
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has also been reported. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
Treatment
Consult with a Certified Poison Control Center for up to date guidance and advice.
TREATMENT
Consult with a Certified Poison Control Center for up-to-date
guidance and advice. Management of acute amphetamine intoxication is
largely symptomatic and includes gastric lavage, administration of
activated charcoal, administration of a cathartic, and sedation.
Experience with hemodialysis or peritoneal dialysis is inadequate to
permit recommendation in this regard. Acidification of the urine
increases amphetamine excretion, but is believed to increase risk of
acute renal failure if myoglobinuria is present. If acute, severe
hypertension complicates amphetamine overdosage, administration of
intravenous phentolamine (Bedford Laboratories) has been suggested.
However, a gradual drop in blood pressure will usually result when
sufficient sedation has been achieved.
Chlorpromazine antagonizes
the central stimulant effects of amphetamines and can be used to treat
amphetamine intoxication.
Since much of
the extended-release capsule medication is coated for gradual
release, therapy directed at reversing the effects of the ingested drug
and at supporting the patient should be continued for as long as
overdosage symptoms remain. Saline cathartics are useful for hastening
the evacuation of pellets that have not already released
medication.
DOSAGE AND ADMINISTRATION
Amphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses should be avoided because of the resulting insomnia.
Narcolepsy
Usual dose is 5 to 60 mg per day in divided
doses, depending on the individual patient
response.
Narcolepsy seldom occurs in children under
12 years of age; however, when it
does, dextroamphetamine sulfate may be used.
The suggested initial dose for patients aged 6 to 12 is
5 mg daily; daily dose may be raised in increments of
5 mg at weekly intervals until an optimal response is
obtained. In patients 12 years of age and older, start
with 10 mg daily; daily dosage may be raised in
increments of 10 mg at weekly intervals until an
optimal response is obtained. If bothersome adverse reactions
appear (e.g., insomnia or anorexia), dosage should be
reduced, extended-release capsules may be used for
once-a-day dosage wherever appropriate.
Attention Deficit Disorder with Hyperactivity
The extended-release capsule
formulation is not recommended for pediatric patients younger
than 6 years of age.
In pediatric
patients 6 years of age and older, start
with 5 mg once or twice daily; daily dosage may be
raised in increments of 5 mg at weekly intervals until
optimal response is obtained.
Only in rare cases will it be
necessary to exceed a total of 40 mg per
day. Extended-release capsules may be used
for once-a-day dosage wherever appropriate.
Where possible,
drug administration should be interrupted occasionally to
determine if there is a recurrence of behavioral symptoms
sufficient to require continued therapy.
HOW SUPPLIED
Dextroamphetamine Sulfate Extended-Release Capsules are supplied as follows:
Dextroamphetamine Sulfate
Extended-Release Capsules, 5 mg: Small, spherical, light
orange pellets and dark orange pellets contained in a size #4 gelatin
capsule. Capsules are imprinted “327” on the yellow cap, and “CP” and “5 mg” on the body.
Bottles of 90 (NDC: 54505-327-09)
Dextroamphetamine Sulfate
Extended-Release Capsules, 10 mg: Small, spherical, light
orange pellets and dark orange pellets contained in a size #4 gelatin
capsule. Capsules are imprinted “328” on the orange cap, and “CP” and “10 mg” on the body.
Bottles of 90 (NDC: 54505-328-09)
Dextroamphetamine Sulfate
Extended-Release Capsules, 15 mg: Small, spherical, light
orange pellets and dark orange pellets contained in a size #3 gelatin
capsule. Capsules are imprinted “329” on the red cap, and “CP” and “15 mg” on the body.
Bottles of 90 (NDC: 54505-329-09)
Store at controlled room temperature between 20° to 25°C (68° to 77°F)[See USP].
Dispense in a tight, light-resistant container.
Manufactured by:
Catalent Pharma Solutions
Winchester, KY
40391
for:
Lineage Therapeutics Inc.
Horsham, PA 19044
LB# 822-07 Rev. March, 2017
For additional copies of the printed patient information/medication guide, please visit www.lineagetherapeutics.com or contact us at 1-888-894-6528.
MEDICATION GUIDE
CII
Dextroamphetamine Sulfate Extended-Release Capsules
Read the Medication Guide that comes with Dextroamphetamine Sulfate Extended-Release Capsules before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your or your child’s treatment with Dextroamphetamine Sulfate Extended-Release Capsules.
What is the most important information
I should know about Dextroamphetamine Sulfate
Extended-Release Capsules?
The following have been
reported with use of Dextroamphetamine Sulfate
Extended-Release Capsules and other stimulant
medicines.
1. Heart-related problems:
- Sudden death in patients who have heart problems or heart defects
- Stroke and heart attack in adults
- Increased blood pressure and heart rate
Tell your doctor if you or your child have any heart
problems, heart defects, high blood pressure, or a family
history of these problems.
Your doctor should check you or
your child carefully for heart problems before starting
Dextroamphetamine Sulfate Extended-Release Capsules.
Your
doctor should check your or your child's blood pressure
and heart rate regularly during treatment with Dextroamphetamine
Sulfate Extended-Release Capsules.
Call your doctor right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking Dextroamphetamine Sulfate Extended-Release Capsules.
2. Mental (Psychiatric) problems:
All Patients
- new or worse behavior and thought problems
- new or worse bipolar illness
- new or worse aggressive behavior or hostility
Children and Teenagers
- new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your doctor right away if you or your child have any new or worsening mental symptoms or problems while taking Dextroamphetamine Sulfate Extended-Release Capsules, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]:
- fingers or toes may feel numb, cool, painful
- fingers or toes may change color from pale, to blue, to red
Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your doctor right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking dextroamphetamine sulfate.
What are Dextroamphetamine Sulfate Extended-Release Capsules?
Dextroamphetamine Sulfate Extended-Release
Capsules are a central nervous system stimulant
prescription medicine. It is used for
the treatment of Attention-Deficit Hyperactivity Disorder
(ADHD).
Dextroamphetamine Sulfate
Extended-Release Capsules may help increase attention
and decrease impulsiveness and hyperactivity in patients with
ADHD.
Dextroamphetamine Sulfate Extended-Release
Capsules should be used as a part of a total treatment
program for ADHD that may include counseling or other
therapies.
Dextroamphetamine Sulfate Extended-Release
Capsules are also used in the treatment of a sleep
disorder called narcolepsy.
Dextroamphetamine Sulfate is a federally controlled substance (CII) because it can be abused or lead to dependence. Keep Dextroamphetamine Sulfate Extended-Release Capsules in a safe place to prevent misuse and abuse. Selling or giving away Dextroamphetamine Sulfate Extended-Release Capsules may harm others and is against the law.
Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take Dextroamphetamine
Sulfate Extended-Release Capsules?
Dextroamphetamine Sulfate
Extended-Release Capsules should not be taken if you or your
child:
- Have heart disease or hardening of the arteries
- Have moderate to severe high blood pressure
- Have hyperthyroidism
- Have an eye problem called glaucoma
- Are very anxious, tense, or agitated
- Have a history of drug abuse
- Are taking or have taken within the past 14 days an antidepression medicine called a monoamine oxidase inhibitor or MAOI
- Is sensitive to, allergic to, or had a reaction to other stimulant medicines
Dextroamphetamine Sulfate Extended-Release Capsules are not recommended for use in children younger than 6 years old.
Dextroamphetamine Sulfate Extended-Release Capsules may not be right for you or your child. Before starting Dextroamphetamine Sulfate Extended-Release Capsules tell your or your child’s doctor about all health conditions (or a family history of) including:
- Heart problems, heart defects, high blood pressure
- Mental problems including psychosis, mania, bipolar illness, or depression
- Tics or Tourette’s syndrome
- Thyroid problems
- Seizures or have had an abnormal brain wave test (EEG)
- Circulation problems in fingers and toes
Tell your doctor if you or your child is pregnant, planning to become pregnant, or breastfeeding.
Can Dextroamphetamine Sulfate Extended-Release Capsules be taken with other medicines?
Tell your doctor about all of the medicines that you or your child take including prescription and nonprescription medicines, vitamins, and herbal supplements. Dextroamphetamine Sulfate Extended-Release Capsules and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking Dextroamphetamine Sulfate Extended-Release Capsules.
Your doctor will decide whether Dextroamphetamine Sulfate Extended-Release Capsules can be taken with other medicines.
Especially tell your doctor if you or your child takes:
- Anti-depression medicines including MAOIs
- Blood pressure medicines
- Antacids
- Seizure medicines
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking Dextroamphetamine Sulfate Extended-Release Capsules without talking to your doctor first.
How should Dextroamphetamine Sulfate Extended-Release Capsules be taken?
- Take Dextroamphetamine Sulfate Extended-Release Capsules exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
- Dextroamphetamine Sulfate Extended-Release Capsules comes as a capsule.
- Dextroamphetamine Sulfate Extended-Release Capsules are usually taken once a day in the morning. Dextroamphetamine Sulfate Extended-Release Capsules are extended release capsules. It releases medicine into your body throughout the day.
- From time to time, your doctor may stop treatment with Dextroamphetamine Sulfate Extended-Release Capsules for a while to check ADHD symptoms.
- Your doctor may do regular checks of the blood, heart, and blood pressure while taking Dextroamphetamine Sulfate Extended-Release Capsules. Children should have their height and weight checked often while taking Dextroamphetamine Sulfate Extended-Release Capsules. Treatment with Dextroamphetamine Sulfate Extended-Release Capsules may be stopped if a problem is found during these check-ups.
- If you or your child takes too much Dextroamphetamine Sulfate Extended-Release Capsules or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of
Dextroamphetamine Sulfate Extended-Release
Capsules?
See “What is the most
important information I should know about Dextroamphetamine
Sulfate Extended-Release Capsules?” for
information on reported heart and mental problems.
Other serious side effects include:
- Slowing of growth (height and weight) in children
- Seizures, mainly in patients with a history of seizures
- Eyesight changes or blurred vision
Common side effects include:
- Fast heartbeat
- Decreased appetite
- Tremors
- Headache
- Trouble sleeping
- Dizziness
- Stomach upset
- Weight loss
- Dry mouth
Dextroamphetamine Sulfate Extended-Release Capsules may affect your or your child’s ability to drive or do other dangerous activities.
Talk to your doctor if you or your child has side effects that are bothersome or do not go away. This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information. Call you doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Dextroamphetamine Sulfate Extended-Release Capsules?
- Store Dextroamphetamine Sulfate Extended-Release Capsules in a safe place at room temperature, 68° to 77°F (20° to 25°C). [See USP]. Protect from light.
- Keep Dextroamphetamine Sulfate Extended-Release Capsules and all medicines out of the reach of children.
General information about
Dextroamphetamine Sulfate Extended-Release
Capsules
Medicines are sometimes prescribed for purposes other
than those listed in a Medication Guide. Do not
use Dextroamphetamine Sulfate Extended-Release Capsules
for a condition for which it was not prescribed. Do not
give Dextroamphetamine Sulfate Extended-Release
Capsules to other people, even if they have the same condition.
It may harm them and it is against the law.
This Medication Guide summarizes the most important information about Dextroamphetamine Sulfate Extended-Release Capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Dextroamphetamine Sulfate Extended-Release Capsules that was written for healthcare professionals. For more information about Dextroamphetamine Sulfate Extended-Release Capsules, please contact Lineage Therapeutics at 1-888-894-6528 or visit www.lineagetherapeutics.com.
What are the ingredients in Dextroamphetamine Sulfate Extended-Release Capsules?
Active Ingredient: Dextroamphetamine sulfate
Inactive Ingredients: Cetyl alcohol, D&C Yellow No. 10, dibutyl sebacate, ethylcellulose, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, hypromellose, polyethylene glycol, povidone, sodium lauryl sulfate, and sugar spheres and trace amounts of other inactive ingredients.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by
Catalent Pharma
Solutions
Winchester, KY 40391
for
Lineage Therapeutics Inc.
Horsham, PA 19044
LB# 823-04 Rev. October, 2013
For additional copies of the printed patient information/medication guide, please visit www.lineagetherapeutics.com or contact us at 1-888-894-6528.
PRINCIPAL DISPLAY PANEL - 5 mg BOTTLE LABEL
5 mg
NDC: 54505-327-09
DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE CAPSULESCII

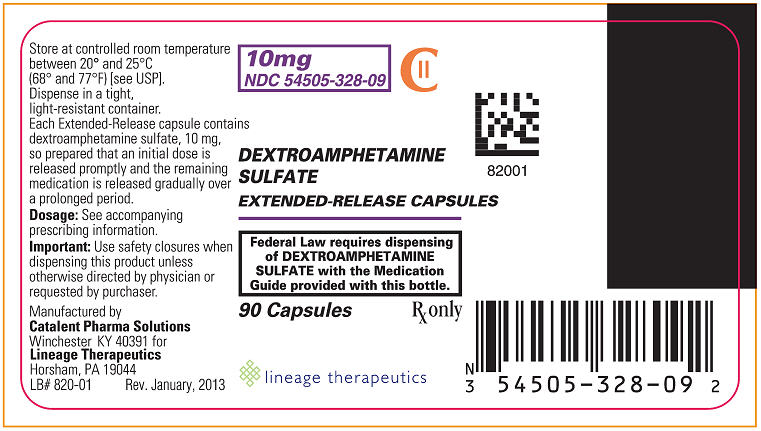
PRINCIPAL DISPLAY PANEL - 10 mg BOTTLE LABEL
10 mg
NDC: 54505-328-09
DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE CAPSULESCII
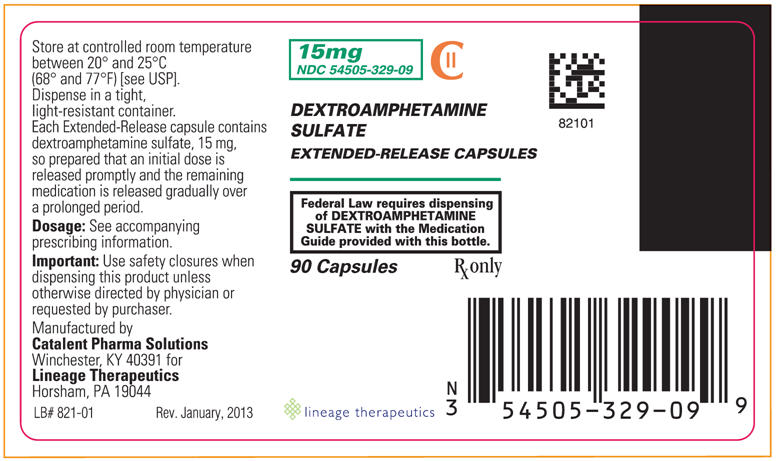
PRINCIPAL DISPLAY PANEL - 15 mg BOTTLE LABEL
15 mg
NDC: 54505-329-09
DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE CAPSULESCII
| DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE
dextroamphetamine sulfate capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE
dextroamphetamine sulfate capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| DEXTROAMPHETAMINE SULFATE
EXTENDED-RELEASE
dextroamphetamine sulfate capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Lineage Therapeutics Inc (078677620) |