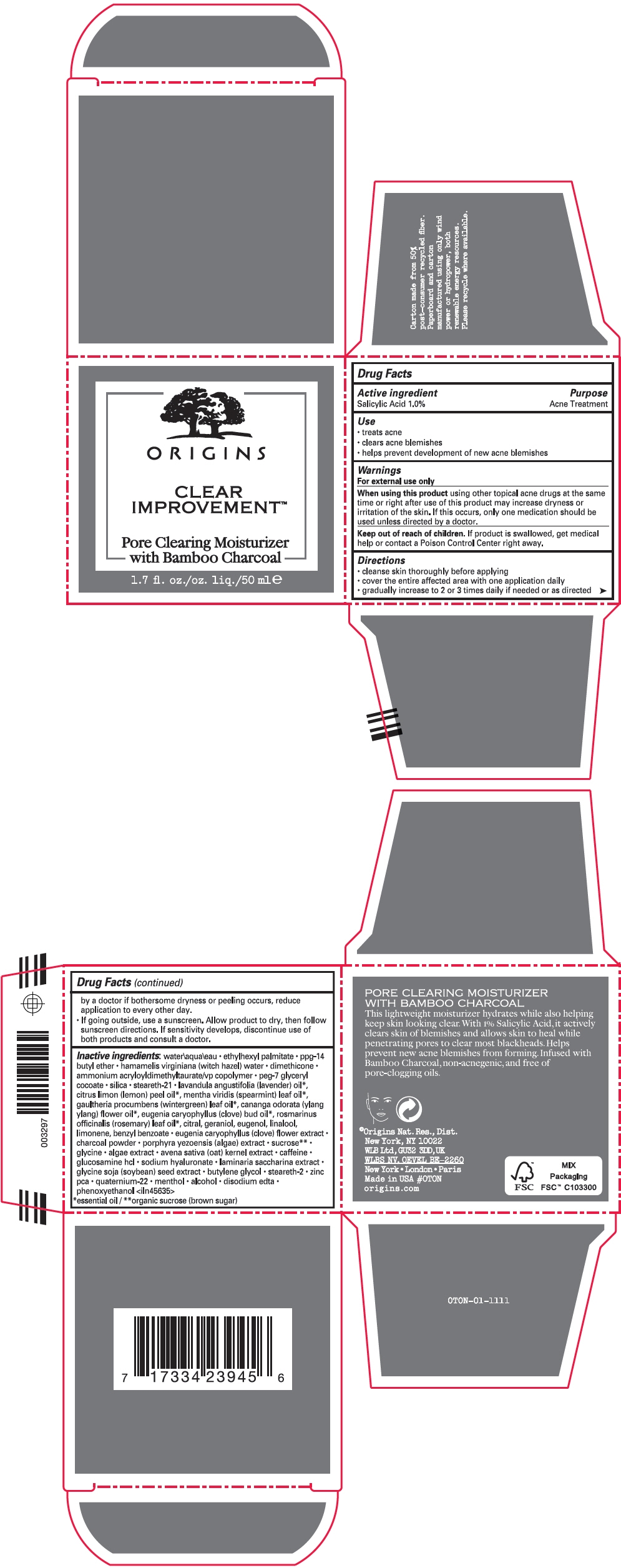
CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER- salicylic acid lotion
CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER by
Drug Labeling and Warnings
CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER by is a Otc medication manufactured, distributed, or labeled by ORIGINS NATURAL RESOURCES INC., Estee Lauder Companies Inc., PALC, Estee Lauder Cosmetics Ltd., Whitman Laboratories Ltd., Estee Lauder N.V., The Estee Lauder Inc, NORTHTEC INC, PADC 1. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- cleanse skin thoroughly before applying
- cover the entire affected area with one application daily
- gradually increase to 2 or 3 times daily if needed or as directed by a doctor if bothersome dryness or peeling occurs, reduce application to every other day.
- If going outside, use a sunscreen. Allow product to dry, then follow sunscreen directions. If sensitivity develops, discontinue use of both products and consult a doctor.
-
Inactive ingredients
water\aqua\eau ethylhexyl palmitate ppg-14 butyl ether hamamelis virginiana (witch hazel) water dimethicone ammonium acryloyldimethyltaurate/vp copolymer peg-7 glyceryl cocoate silica steareth-21 lavandula angustifolia (lavender) oil1, citrus limon (lemon) peel oil1, mentha viridis (spearmint) leaf oil1, gaultheria procumbens (wintergreen) leaf oil1, cananga odorata (ylang ylang) flower oil1, eugenia caryophyllus (clove) bud oil1, rosmarinus officinalis (rosemary) leaf oil1, citral, geraniol, eugenol, linalool, limonene, benzyl benzoate eugenia caryophyllus (clove) flower extract charcoal powder porphyra yezoensis (algae) extract sucrose2 glycine algae extract avena sativa (oat) kernel extract caffeine glucosamine hcl sodium hyaluronate laminaria saccharina extract glycine soja (soybean) seed extract butylene glycol steareth-2 zinc pca quaternium-22 menthol alcohol disodium edta phenoxyethanol <iln45635>
- 1 essential oil
- 2 organic sucrose (brown sugar)
- PRINCIPAL DISPLAY PANEL - 50 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59427-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic acid (UNII: O414PZ4LPZ) (Salicylic acid - UNII:O414PZ4LPZ) Salicylic acid 0.01015 g in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) ethylhexyl palmitate (UNII: 2865993309) ppg-14 butyl ether (UNII: R199TJT95T) witch hazel (UNII: 101I4J0U34) dimethicone (UNII: 92RU3N3Y1O) ammonium acryloyldimethyltaurate/vp copolymer (UNII: W59H9296ZG) peg-7 glyceryl cocoate (UNII: VNX7251543) silicon dioxide (UNII: ETJ7Z6XBU4) steareth-21 (UNII: 53J3F32P58) lavender oil (UNII: ZBP1YXW0H8) lemon oil (UNII: I9GRO824LL) spearmint oil (UNII: C3M81465G5) methyl salicylate (UNII: LAV5U5022Y) cananga oil (UNII: 8YOY78GNNX) clove oil (UNII: 578389D6D0) rosemary oil (UNII: 8LGU7VM393) citral (UNII: T7EU0O9VPP) geraniol (UNII: L837108USY) eugenol (UNII: 3T8H1794QW) linalool, (+/-)- (UNII: D81QY6I88E) limonene, (+)- (UNII: GFD7C86Q1W) benzyl benzoate (UNII: N863NB338G) clove (UNII: K48IKT5321) activated charcoal (UNII: 2P3VWU3H10) phymatolithon calcareum (UNII: 6J1M3WA0ZK) sucrose (UNII: C151H8M554) glycine (UNII: TE7660XO1C) oat (UNII: Z6J799EAJK) caffeine (UNII: 3G6A5W338E) glucosamine hydrochloride (UNII: 750W5330FY) hyaluronate sodium (UNII: YSE9PPT4TH) saccharina latissima (UNII: 68CMP2MB55) soybean (UNII: L7HT8F1ZOD) butylene glycol (UNII: 3XUS85K0RA) steareth-2 (UNII: V56DFE46J5) zinc pidolate (UNII: C32PQ86DH4) quaternium-22 (UNII: MXO138JCBP) menthol, unspecified form (UNII: L7T10EIP3A) alcohol (UNII: 3K9958V90M) edetate disodium (UNII: 7FLD91C86K) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59427-015-01 1 in 1 CARTON 09/23/2019 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 09/23/2019 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(59427-015) , REPACK(59427-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(59427-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(59427-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(59427-015) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(59427-015) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(59427-015) , REPACK(59427-015) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(59427-015) , REPACK(59427-015) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(59427-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(59427-015)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.