Best Health Sore Throat Relief Cherry
Best Health Sore Throat Relief Cherry Flavor by
Drug Labeling and Warnings
Best Health Sore Throat Relief Cherry Flavor by is a Otc medication manufactured, distributed, or labeled by Bestco, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BEST HEALTH SORE THROAT RELIEF CHERRY FLAVOR- benzocaine, menthol lozenge
Bestco, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Best Health Sore Throat Relief Cherry
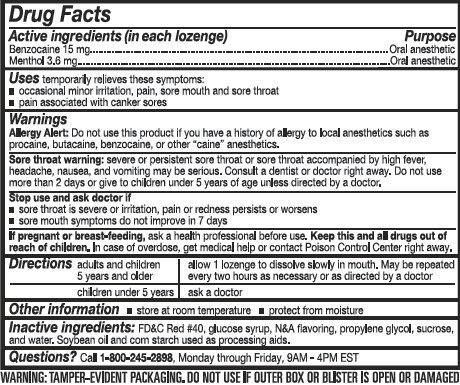
Uses
temporarily relieves these symptoms:
- occasional minor irritation, pain, sore mouth and sore throat
- pain associated with canker sores
Warnings
Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or any other "caine" anesthetics.
Directions
-
adults and children 5 years and older: allow 1 lozenge to dissolve slowly in the mouth. May be repeated every 2 hours as needed or as directed by a doctor or dentist.
-
children under 5 years: ask a doctor
Inactive ingredients
FD C Red 40, glucose syrup, N A Flavoring, propylene glycol, sucrose, and water. Soybean oil and corn starch used as processing aids.
Best Health Sore Throat Lozenges Cherry Flavor 18 Count (52642-010-18)
Strong, Effective and Affordable for your.....
Best Health
Sore Throat
Relief Lozenges
MENTHOL BENZOCAINE
Fast Acting ORAL ANESTHETIC
Fast, soothing, temporary
relief of minor sore throat
pain and Irritation
Compare to active ingredients in Cepacol
18 LOZENGES
Manufactured in the USA by:
BestSweet Inc.
Mooresville, NC 28115
2012 BestSweet Inc.
This product is not manufactured or distributed
by Reckftt Bencldser owner af the registered
trademark Cepacol.

| BEST HEALTH SORE THROAT RELIEF CHERRY FLAVOR
benzocaine, menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bestco, Inc. (002149136) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bestco, Inc. | 002149136 | manufacture(52642-010) | |