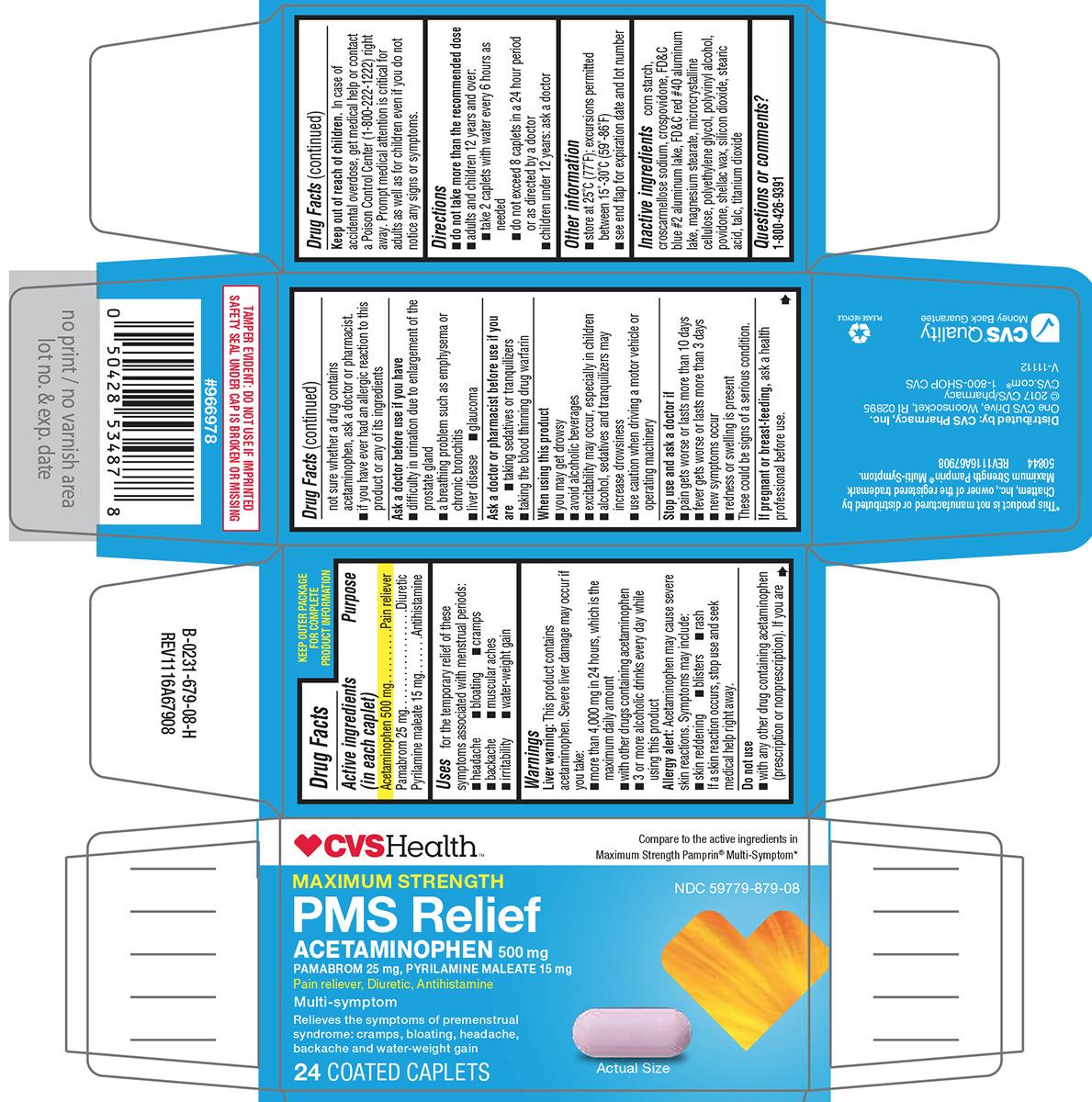
PMS RELIEF MAXIMUM STRENGTH- acetaminophen, pamabrom, pyrilamine maleate tablet, film coated
PMS Relief by
Drug Labeling and Warnings
PMS Relief by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- liver disease
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- you may get drowsy
- avoid alcoholic beverages
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
CVSHealth™
Compare to the active ingredients in Maximum Strength Pamprin® Multi-Symptom*
NDC: 59779-879-08
MAXIMUM STRENGTH
PMS Relief
ACETAMINOPHEN 500 mg
PAMABROM 25 mg, PYRILAMINE MALEATE 15 mg
Pain reliever, Diuretic, AntihistamineMulti-symptom
Relieves the symptoms of premenstrual
syndrome: cramps, bloating, headache,
backache and water-weight gain24 COATED CAPLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Chattem, Inc., owner of the registered trademark Maximum Strength Pamprin® Multi-Symptom.
50844 REV1116A67908
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
©2017 CVS/pharmacy
CVS.com® 1-800-SHOP CVSV-11112
CVS®Quality
Money Back Guarantee
CVS 44-679
-
INGREDIENTS AND APPEARANCE
PMS RELIEF MAXIMUM STRENGTH
acetaminophen, pamabrom, pyrilamine maleate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59779-879 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg PAMABROM (UNII: UA8U0KJM72) (BROMOTHEOPHYLLINE - UNII:FZG87K1MQ6) PAMABROM 25 mg PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 15 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) CROSPOVIDONE (UNII: 2S7830E561) SHELLAC (UNII: 46N107B71O) Product Characteristics Color PURPLE Score no score Shape OVAL Size 17mm Flavor Imprint Code 44;679 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59779-879-27 1 in 1 CARTON 01/13/2015 1 32 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 59779-879-08 1 in 1 CARTON 01/13/2015 2 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 01/13/2015 Labeler - CVS Pharmacy (062312574) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(59779-879) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(59779-879) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(59779-879) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(59779-879) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(59779-879)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.