These highlights do not include all the information needed to use BREXPIPRAZOLE TABLETS safely and effectively. See full prescribing information for BREXPIPRAZOLE TABLETS. BREXPIPRAZOLE tablets, for oral use Initial U.S. Approval: 2015
Brexpiprazole by
Drug Labeling and Warnings
Brexpiprazole by is a Prescription medication manufactured, distributed, or labeled by Camber Pharmaceuticals, Inc., Hetero Labs Limited Unit V. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BREXPIPRAZOLE
- brexpiprazole tablet, film coated
Camber Pharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BREXPIPRAZOLE TABLETS safely and effectively. See full prescribing information for BREXPIPRAZOLE TABLETS.
BREXPIPRAZOLE tablets, for oral use Initial U.S. Approval: 2015
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS
|
|
Indication |
Starting Dosage |
Recommended Target Dosage |
Maximum Dosage |
|
MDD Adults (2.2) |
0.5 mg/day or 1 mg/day |
2 mg/day |
3 mg/day |
|
Schizophrenia Adults (2.3) |
1 mg/day |
2 to 4 mg/day |
4 mg/day |
Moderate to Severe Hepatic Impairment:Maximum recommended dosage is 2 mg once daily for patients with MDD and 3 mg once daily for patients with schizophrenia. (
2.5)
CrCl<60 mL/minute:Maximum recommended dosage is 2 mg once daily for patients with MDD and 3 mg once daily for patients with schizophrenia. (2.6)
See Full Prescribing Information for dosage modifications for CYP2D6 poor metabolizers and for concomitant use with CYP inhibitors or inducers. (2.7)
DOSAGE FORMS AND STRENGTHS
Tablets: 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, and 4 mg ( 3)
CONTRAINDICATIONS
Known hypersensitivity to brexpiprazole tablets or any of its components ( 4)
WARNINGS AND PRECAUTIONS
Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis:Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack) (
5.3)
Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring. (
5.4)
Tardive Dyskinesia: Discontinue if clinically appropriate. (
5.5)
Metabolic Changes:Monitor for hyperglycemia/diabetes mellitus, dyslipidemia and weight gain. (
5.6)
Pathological Gambling and Other Compulsive Behaviors:Consider dose reduction or discontinuation. (
5.7)
Leukopenia, Neutropenia, and Agranulocytosis:Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing brexpiprazole if a clinically significant decline in WBC occurs in absence of other causative factors. (
5.8)
Orthostatic Hypotension and Syncope:Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope. (
5.9)
Seizures:Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. (
5.11)
ADVERSE REACTIONS
Most common adverse reactions in adults were (
6.1):
MDD:Weight increased, somnolence, and akathisia (≥5% and at least twice the rate for placebo)
Schizophrenia:Weight increased (≥4% and at least twice the rate for placebo)
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088
(www.fda.gov/medwatch).
DRUG INTERACTIONS
| Factors
| Dosage Adjustments forBrexpiprazole (2.7)
|
| Strong CYP2D6* or CYP3A4
inhibitors | Administer half of recommended dosage.
|
| Strong/moderate CYP2D6 with Strong/moderate CYP3A4 inhibitors
| Administer a quarter of the recommended dosage.
|
| Known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors
| Administer a quarter of the recommended dosage.
|
| Strong CYP3A4 inducers
| Double the recommended dosage and further adjust based on clinical response.
|
* Brexpiprazole may be administered without dosage adjustment in patients with MDD when administered with strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine). (7)
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure (
8.1)
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti
®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2024
FULL PRESCRIBING INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderlypatients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Brexpiprazole is not approved for the treatment of patients with dementia-related psychosis
[see
Warnings and Precautions (5.1)].
Suicidal Thoughts and Behaviors
Antidepressants increased the risk of suicidal thoughts and behaviors in patients aged 24 years and younger in short-term studies. Monitor closely for clinical worsening and for emergence of suicidal thoughts and behaviors. The safety and effectiveness of brexpiprazole have not been established in pediatric patients with MDD
[see
Warnings and Precautions (5.2),
Use in Specific Populations (8.4)].
1 INDICATIONS AND USAGE
Brexpiprazole tablets are indicated for:
Adjunctive treatment of major depressive disorder (MDD) in adults
Treatment of schizophrenia in adults
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information
Administer brexpiprazole tablets orally, once daily with or without food [see Clinical Pharmacology (12.3)]
2.2 Recommended Dosage for Adjunctive Treatment of Major Depressive Disorder (Adults)
The recommended starting brexpiprazole tablets dosage for the adjunctive treatment of MDD in adults is 0.5 mg or 1 mg once daily. Titrate to 1 mg once daily, then titrate to the target dosage of 2 mg once daily (based on the patient’s clinical response and tolerability, increase the dosage at weekly intervals). The maximum recommended daily dosage is 3 mg. Periodically reassess to determine the continued need and appropriate dosage for treatment.
2.3 Recommended Dosage for Schizophrenia (Adults)
Adults
The recommended starting brexpiprazole dosage for the treatment of schizophrenia in adults is 1 mg once daily on Days 1 to 4. Titrate to 2 mg oncedailyon Day 5 through Day 7. On Day 8, the dosage can be increased to the maximum recommended daily dosage of 4 mg based on clinical response and tolerability. The recommended target dosage is 2 mg to 4 mg once daily.
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti® (brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.5 Recommended Dosage in Patients with Hepatic Impairment
The maximum recommended dosage in patients with moderate to severe hepatic impairment (Child-Pugh score ≥7) is
[see
Use in Specific Populations (8.7),
Clinical Pharmacology (12.3)].
2 mg once daily in patients with MDD and
3 mg orally once daily in patients with schizophrenia
2.6 Recommended Dosage in Patients with Renal Impairment
The maximum recommended dosage in patients with creatinine clearance CrCl<60 mL/minute is
[see
Use in Specific Populations (8.8),
Clinical Pharmacology (12.3)].
2 mg orally once daily in patients with MDD and
3 mg orally once daily in patients with schizophrenia
2.7 Dosage Modifications for CYP2D6 Poor Metabolizers and for Concomitant Use with CYP Inhibitors or Inducers
Dosage modifications are recommended in patients who are known cytochrome P450 (CYP) 2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors, CYP2D6 inhibitors, or strong CYP3A4 inducers (see Table 1). If the concomitant drug is discontinued, adjust the brexpiprazole tablets dosage to its original level. If the concomitant CYP3A4 inducer is discontinued, reduce the brexpiprazole tablets dosage to the original level over 1 to 2 weeks
[see
Drug Interactions (7.1),
Clinical Pharmacology (12.3)].
Table 1 Dosage Modifications of Brexpiprazole Tablets for CYP2D6 Poor Metabolizers and for Concomitant Use with CYP3A4 Inhibitors, CYP2D6 Inhibitors, or CYP3A4 Inducers
| Factors
| Adjusted Brexpiprazole Tablets Dosage
|
| CYP2D6 Poor Metabolizers
|
|
| CYP2D6 poor metabolizers
| Administer half of the recommended dosage.
|
| Known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors
| Administer a quarter of the recommended dosage.
|
| Patients Taking CYP2D6 Inhibitors and/or CYP3A4 Inhibitors
|
|
| Strong CYP2D6 inhibitors*
| Administer half of the recommended dosage.
|
| Strong CYP3A4 inhibitors
| Administer half of the recommended dosage.
|
| Strong/moderate CYP2D6 inhibitors with strong/moderate CYP3A4 inhibitors
| Administer a quarter of the recommended dosage.
|
| Patients Taking CYP3A4 Inducers
|
|
| Strong CYP3A4 inducers
| Double the recommended dosage over 1 to 2 weeks.
|
*In the clinical studies examining the use of brexpiprazole tablets for the adjunctive treatment of MDD, dosage was not adjusted for strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine). Thus, CYP considerations are already factored into general dosing recommendations, and brexpiprazole tablets may be administered without dosage adjustment in patients with MDD.
3 DOSAGE FORMS AND STRENGTHS
Brexpiprazole tablets are available in 6 strengths:
0.25 mg tablets are round shape grey colored film coated tablets with "B8" on one side and "H" on other side.
0.5 mg tablets are round shape pink colored film coated tablets with "B9" on one side and "H" on other side.
1 mg tablets are round shape beige colored film coated tablets with "B10" on one side and "H" on other side.
2 mg tablets are round shape blue colored film coated tablets with "B11" on one side and "H" on other side.
3 mg tablets are round shape red colored film coated tablets with "B12" on one side and "H" on other side.
4 mg tablets are round shape white to off white colored film coated tablets with "B13" on one side and "H" on other side.
4 CONTRAINDICATIONS
Brexpiprazole tablets are contraindicated in patients with a known hypersensitivity to brexpiprazole or any of its components. Reactions have included rash, facial swelling, urticaria, and anaphylaxis.
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in the drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Brexpiprazole is not approved for the treatment of patients with dementia-related psychosis
[see
Boxed Warning, Warnings and Precautions (5.3)].
5.2 Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and over 4400 pediatric patients, the incidence of suicidal thoughts and behaviors in patients 24 years of age and younger was greater in antidepressant-treated patients than in placebo-treated patients. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 2.
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about antidepressant drug effect on suicide.
Table 2 Risk Differences of the Number of Patients with Suicidal Thoughts or Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric* and Adult Patients
| Age Range
(years) | Drug-Placebo Difference in Number of Patients with Suicidal Thoughts or Behaviors per 1000 Patients Treated
|
|
| Increases Compared to Placebo
|
| <18
| 14 additional patients
|
| 18 to 24
| 5 additional patients
|
|
| Decreases Compared to Placebo
|
| 25 to 64
| 1 fewer patient
|
| ≥65
| 6 fewer patients
|
* Brexpiprazole is not approved in pediatric patients with MDD.
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing brexpiprazole, in patients whose depression is persistently worse or who are experiencing emergent suicidal thoughts or behaviors.
5.3 Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials in elderly patients with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. Brexpiprazole is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.1)].
5.4 Neuroleptic Malignant Syndrome (NMS)
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with administration of antipsychotic drugs, including brexpiprazole.
Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
If NMS is suspected, immediately discontinue brexpiprazole and provide intensive symptomatic treatment and monitoring.
5.5 Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. The risk appears to be highest among the elderly, especially elderly women, but it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drugs differ in their potential to cause tardive dyskinesia is unknown.
The risk of tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the cumulative dose increases. The syndrome can develop after relatively brief treatment periods, at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of tardive dyskinesia is unknown.
Given these considerations, brexpiprazole should be prescribed in a manner most likely to reduce the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that 1) is known to respond to antipsychotic drugs and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment needed to produce a satisfactory clinical response. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient treated with brexpiprazole, drug discontinuation should be considered. However, some patients may require treatment with brexpiprazole despite the presence of the syndrome.
5.6 Metabolic Changes
Atypical antipsychotic drugs, including brexpiprazole, have caused metabolic changes including hyperglycemia, diabetes mellitus, dyslipidemia, and body weight gain. Although all of the drugs in the class to date have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia and diabetes mellitus, in some cases extreme and associated with diabetic ketoacidosis hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia in patients treated with brexpiprazole. Assess fasting plasma glucose before or soon after initiation of antipsychotic medication and monitor periodically during long-term treatment.
Adjunctive Treatment of Major Depressive Disorder:In the 6-week placebo-controlled, fixed-dose clinical studies in adult patients with MDD, the proportions of patients with shifts in fasting glucose from normal (<100 mg/dL) to high (≥126 mg/dL) and borderline (≥100 and <126 mg/dL) to high were similar in patients treated with brexpiprazole and placebo. In the long-term, open-label depression studies, 5% of adult patients with normal baseline fasting glucose experienced a shift to high while taking brexpiprazole plus an antidepressant (ADT); 25% of patients with borderline fasting glucose experienced shifts to high. Combined, 9% of patients with normal or borderline fasting glucose experienced shifts to high fasting glucose during the long-term depression studies.
Schizophrenia (Adults):In the 6-week placebo-controlled, fixed-dose clinical studies in adult patients with schizophrenia, the proportions of patients with shifts in fasting glucose from normal (<100 mg/dL) to high (≥126 mg/dL) or borderline (≥100 and <126 mg/dL) to high were similar in patients treated with brexpiprazole and placebo. In the long-term, open-label schizophrenia studies, 8% of adult patients with normal baseline fasting glucose experienced a shift from normal to high while taking brexpiprazole; 17% of patients with borderline fasting glucose experienced shifts from borderline to high. Combined, 10% of patients with normal or borderline fasting glucose experienced shifts to high fasting glucose during the long-term schizophrenia studies.
Dyslipidemia
Atypical antipsychotics cause adverse alterations in lipids. Before or soon after initiation of antipsychotic medication, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
Adjunctive Treatment of Major Depressive Disorder:In the 6-week placebo-controlled, fixed-dose clinical studies in adult patients with MDD, changes in fasting total cholesterol, LDL cholesterol, and HDL cholesterol were similar in brexpiprazole- and placebo-treated patients. Table 3 shows the proportions of patients with changes in fasting triglycerides.
Table 3 Change in Fasting Triglycerides in the 6-Week Placebo-Controlled, Fixed-Dose MDD Studies
| Proportion of Patients with Shifts Baseline to Post-Baseline
|
||||
| Triglycerides
| Placebo
| 1 mg/day
| 2 mg/day
| 3 mg/day
|
| Normal to High
(<150 mg/dL to ≥200 and <500 mg/dL) | 6%
(15/257)* | 5%
(7/145)* | 13%
(15/115)* | 9%
(13/150)* |
| Normal/Borderline to Very High
(<200 mg/dL to ≥500 mg/dL) | 0%
(0/309)* | 0%
(0/177)* | 0.7%
(1/143)* | 0%
(0/179)* |
* denotes n/N where N=the total number of patients who had a measurement at baseline and at least one post-baseline result
n=the number of patients with shift
In the long-term, open-label depression studies, shifts in baseline fasting cholesterol from normal to high were reported in 9% (total cholesterol), 3% (LDL cholesterol), and shifts in baseline from normal to low were reported in 14% (HDL cholesterol) of patients taking brexpiprazole. Of patients with normal baseline triglycerides, 17% experienced shifts to high, and 0.2% experienced shifts to very high. Combined, 0.6% of patients with normal or borderline fasting triglycerides experienced shifts to very high fasting triglycerides during the long-term depression studies.
Schizophrenia (Adults): In the 6-week placebo-controlled, fixed-dose clinical studies in adult patients with schizophrenia, changes in fasting total cholesterol, LDL cholesterol, and HDL cholesterol were similar in brexpiprazole- and placebo-treated patients. Table 4 shows the proportions of patients with changes in fasting triglycerides.
Table 4 Change in Fasting Triglycerides in the 6-Week Placebo-Controlled, Fixed-Dose Schizophrenia Studies in Adult Patients
| Proportion of Patients with Shifts Baseline to Post-Baseline
|
||||
| Triglycerides
| Placebo
| 1 mg/day
| 2 mg/day
| 4 mg/day
|
| Normal to High
(<150 mg/dL to ≥200 and <500 mg/dL) | 6%
(15/253)* | 10%
(7/72)* | 8%
(19/232)* | 10%
(22/226)* |
| Normal/Borderline to Very High
(<200 mg/dL to ≥500 mg/dL) | 0%
(0/303)* | 0%
(0/94)* | 0%
(0/283)* | 0.4%
(1/283)* |
* denotes n/N where N=the total number of patients who had a measurement at baseline and at least one post-baseline result
n=the number of patients with shift
In the long-term, open-label schizophrenia studies in adult patients, shifts in baseline fasting cholesterol from normal to high were reported in 6% (total cholesterol), 2% (LDL cholesterol), and shifts in baseline from normal to low were reported in 17% (HDL cholesterol) of patients taking brexpiprazole. Of patients with normal baseline triglycerides, 13% experienced shifts to high, and 0.4% experienced shifts to very high triglycerides. Combined, 0.6% of patients with normal or borderline fasting triglycerides experienced shifts to very high fasting triglycerides during the long-term schizophrenia studies.
Weight Gain
Weight gain has been observed in patients treated with atypical antipsychotics, including brexpiprazole. Monitor weight at baseline and frequently thereafter.
Adjunctive Treatment of Major Depressive Disorder: Table 6 shows weight gain data at last visit and percentage of adult patients with ≥7% increase in body weight at endpoint from the 6-week placebo-controlled, fixed-dose clinical studies in patients with MDD.
Table 6 Increases in Body Weight in the 6-Week Placebo-Controlled, Fixed-Dose MDD Studies
|
| Placebo
| 1 mg/day
| 2 mg/day
| 3 mg/day
|
|
| n=407
| n=225
| n=187
| n=228
|
| Mean Change from Baseline (kg)at Last Visit
|
||||
| All Patients
| +0.3
| +1.3
| +1.6
| +1.6
|
| Proportion of Patients witha ≥7% Increase in Body Weight (kg) at Any Visit (*n/N)
|
||||
|
| 2%
| 5%
| 5%
| 2%
|
|
| (8/407)*
| (11/225)*
| (9/187)*
| (5/228)*
|
* N=the total number of patients who had a measurement at baseline and at least one post-baseline result
n=the number of patients with a shift ≥7%
In the long-term, open-label depression studies, 4% of patients discontinued due to weight increase. Brexpiprazole was associated with mean change from baseline in weight of 2.9 kg at Week 26 and 3.1 kg at Week 52. In the long-term, open-label depression studies, 30% of patients demonstrated a ≥7% increase in body weight, and 4% demonstrated a ≥7% decrease in body weight.
Schizophrenia (Adults):Table 7 shows weight gain data at last visit and percentage of adult patients with ≥7% increase in body weight at endpoint from the 6-week placebo-controlled, fixed-dose clinical studies in adult patients with schizophrenia.
Table 7 Increases in Body Weight in the 6-Week Placebo-Controlled, Fixed-Dose Schizophrenia Studies in Adult Patients
|
| Placebo
| 1 mg/day
| 2 mg/day
| 4 mg/day
|
|
| n=362
| n=120
| n=362
| n=362
|
| Mean Change from Baseline (kg)at Last Visit
|
||||
| All Patients
| +0.2
| +1.0
| +1.2
| +1.2
|
| Proportion of Patients witha ≥7% Increase in Body Weight (kg) at Any Visit (*n/N)
|
||||
|
| 4%
| 10%
| 11%
| 10%
|
|
| (15/362)*
| (12/120)*
| (38/362)*
| (37/362)*
|
*denotes n/N where N=the total number of patients who had a measurement at baseline and at least one post-baseline result
n=the number of patients with a shift ≥7%
In the long-term, open-label schizophrenia studies in adult patients, 0.6% of patients discontinued due to weight increase. Brexpiprazole was associated with mean change from baseline in weight of 1.3 kg at Week 26 and 2.0 kg at Week 52. In the long-term, open label schizophrenia studies, 20% of patients demonstrated a ≥7% increase in body weight, and 10% demonstrated a ≥7% decrease in body weight.
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti
®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information.
5.7 Pathological Gambling and Other Compulsive Behaviors
Post-marketing case reports suggest that patients can experience intense urges, particularly for gambling, and the inability to control these urges while taking brexpiprazole. Other compulsive urges, reported less frequently, include sexual urges, shopping, eating, or binge eating, and other impulsive or compulsive behaviors. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or intense gambling urges, compulsive sexual urges, compulsive shopping, binge or compulsive eating, or other urges while being treated with brexpiprazole. In some cases, although not all, urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Compulsive behaviors may result in harm to the patient and others if not recognized. Consider dose reduction or stopping the medication if a patient develops such urges.
5.8 Leukopenia, Neutropenia, and Agranulocytosis
Leukopenia and neutropenia have been reported during treatment with antipsychotic agents. Agranulocytosis (including fatal cases) has been reported with other agents in this class.
Possible risk factors for leukopenia and neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug-induced leukopenia or neutropenia. In patients with a pre-existing low WBC or ANC or a history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of brexpiprazole at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue brexpiprazole in patients with absolute neutrophil count <1000/mm
3and follow their WBC until recovery.
5.9 Orthostatic Hypotension and Syncope
Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose titration and when increasing the dose. In the short-term, placebo-controlled clinical studies of brexpiprazole plus ADT in adult patients with MDD, the incidence of orthostatic hypotension-related adverse reactions in brexpiprazole plus ADT-treated patients compared to placebo plus ADT-treated patients included: dizziness (2% versus 2%) and orthostatic hypotension (0.1% versus 0%). In the short-term, placebo-controlled clinical studies of brexpiprazole in adult patients with schizophrenia, the incidence of orthostatic hypotension-related adverse reactions in brexpiprazole-treated patients compared to placebo patients included: dizziness (2% versus 2%), orthostatic hypotension (0.4% versus 0.2%), and syncope (0.1% versus 0%).
Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (e.g., elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medication), patients with known cardiovascular disease (history of myocardial infarction, ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. Brexpiprazole has not been evaluated in patients with a recent history of myocardial infarction or unstable cardiovascular disease. Such patients were excluded from the premarketing clinical studies.
5.10 Falls
Antipsychotics, including brexpiprazole, may cause somnolence, postural hypotension, motor, and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.11 Seizures
Like other antipsychotic drugs, brexpiprazole may cause seizures. This risk is greatest in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in older patients.
5.12 Body Temperature Dysregulation
Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use brexpiprazole with caution in patients who may experience these conditions.
5.13 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Antipsychotic drugs, including brexpiprazole, should be used cautiously in patients at risk for aspiration.
5.14 Potential for Cognitive and Motor Impairment
Brexpiprazole, like other antipsychotics, has the potential to impair judgment, thinking, or motor skills. In the 6-week placebo-controlled clinical studies in patients with MDD, somnolence (including sedation and hypersomnia) was reported in 4% of brexpiprazole plus ADT-treated patients compared to 1% of placebo plus ADT-treated patients.
In the 6-week placebo-controlled clinical studies in adult patients with schizophrenia, somnolence (including sedation and hypersomnia) was reported in 5% of brexpiprazole-treated patients compared to 3% of placebo-treated patients.
Patients should be cautioned about operating hazardous machinery, including motor vehicles, until they are reasonably certain that brexpiprazole therapy does not affect them adversely.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
[see
Boxed Warning, Warnings and Precautions (5.1)]
Suicidal Thoughts and Behaviors in Adolescents and Young Adults
[see
Boxed Warning, Warnings and Precautions (5.2)]
Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis
[see
Warnings and Precautions (5.3)]
Neuroleptic Malignant Syndrome (NMS)
[see
Warnings and Precautions (5.4)]
Tardive Dyskinesia
[see
Warnings and Precautions (5.5)]
Metabolic Changes
[see
Warnings and Precautions (5.6)]
Pathological Gambling and Other Compulsive Behaviors
[see
Warnings and Precautions (5.7)]
Leukopenia, Neutropenia, and Agranulocytosis
[see
Warnings and Precautions (5.8)]
Orthostatic Hypotension and Syncope
[see
Warnings and Precautions (5.9)]
Falls
[see
Warnings and Precautions (5.10)]
Seizures
[see
Warnings and Precautions (5.11)]
Body Temperature Dysregulation
[see
Warnings and Precautions (5.12)]
Dysphagia
[see
Warnings and Precautions (5.13)]
Potential for Cognitive and Motor Impairment
[see
Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adjunctive Treatment in Major Depressive Disorder (MDD)
The safety of brexpiprazole was evaluated in 1054 adult patients (18 to 65 years of age) diagnosed with MDD who participated in two 6-week placebo-controlled, fixed-dose clinical studies in patients with major depressive disorder in which brexpiprazole was administered at doses of 1 mg to 3 mg daily as adjunctive treatment to continued antidepressant therapy; patients in the placebo group continued to receive antidepressant therapy
[see
Clinical Studies (14.1)].
Adverse Reactions Reported as Reasons for Discontinuation of Treatment
A total of 3% (17/643) of brexpiprazole-treated patients and 1% (3/411) of placebo-treated patients discontinued due to adverse reactions.
Adverse Reactions in brexpiprazole Studies for Adjunctive MDD in Adults
Adverse reactions associated with the adjunctive use of brexpiprazole (incidence of 2% or greater and adjunctive brexpiprazole incidence greater than adjunctive placebo) that occurred during acute therapy (up to 6-weeks in patients with MDD) are shown in Table 8.
Table 8 Adverse Reactions in ≥2% of Brexpiprazole-Treated Patients and Greater than Placebo in Pooled 6-Week Placebo-Controlled, Fixed-Dose Adjunctive MDD Studies in Adults (Study 1 and Study 2)
|
|
Placebo (N=411) % | Brexpiprazole
|
|||
| 1 mg/day
(N=226) % | 2 mg/day
(N=188) % | 3 mg/day
(N=229) % | All
Brexpiprazole (N=643) % |
||
| Gastrointestinal Disorders
|
|||||
| Constipation
| 1
| 3
| 2
| 1
| 2
|
| General Disorders and Administration Site Conditions
|
|||||
| Fatigue
| 2
| 3
| 2
| 5
| 3
|
| Infections and Infestations
|
|||||
| Nasopharyngitis
| 2
| 7
| 1
| 3
| 4
|
| Investigations
|
|||||
| Weight Increased
| 2
| 7
| 8
| 6
| 7
|
| Blood Cortisol
Decreased | 1
| 4
| 0
| 3
| 2
|
| Metabolism and Nutrition
|
|||||
| Increased Appetite
| 2
| 3
| 3
| 2
| 3
|
| Nervous System Disorders
|
|||||
| Akathisia
| 2
| 4
| 7
| 14
| 9
|
| Headache
| 6
| 9
| 4
| 6
| 7
|
| Somnolence
| 0.5
| 4
| 4
| 6
| 5
|
| Tremor
| 2
| 4
| 2
| 5
| 4
|
| Dizziness
| 1
| 1
| 5
| 2
| 3
|
| Psychiatric Disorders
|
|||||
| Anxiety
| 1
| 2
| 4
| 4
| 3
|
| Restlessness
| 0
| 2
| 3
| 4
| 3
|
Dose-Related Adverse Reactions in the Adjunctive MDD Studies
In Studies 1 and 2, among the adverse reactions that occurred at ≥2% incidence in the patients treated with brexpiprazole plus ADT, the incidences of akathisia and restlessness increased with increases in dose.
Schizophrenia
Adults
The safety of brexpiprazole was evaluated in 852 adult patients (18 to 65 years of age) diagnosed with schizophrenia who participated in two 6-week placebo-controlled, fixed-dose clinical studies in which brexpiprazole was administered at daily doses of 1 mg, 2 mg, and 4 mg
[see
Clinical Studies (14.2)].
Adverse Reactions Occurring at an Incidence of 2% or More in Patients Treated with brexpiprazole for Schizophrenia
Adverse reactions associated with brexpiprazole (incidence of 2% or greater and brexpiprazole incidence greater than placebo) during short-term (up to 6 weeks) studies in adult patients with schizophrenia are shown in Table 9.
Table 9 Adverse Reactions in ≥2% of Brexpiprazole -Treated Patients and Greater than Placebo in Pooled 6-Week Placebo-Controlled, Fixed-Dose Schizophrenia Studies in Adult Patients (Study 3 and Study 4)
|
|
Placebo (N=368) % | Brexpiprazole
|
|||
| 1 mg/day
(N=120) % | 2 mg/day
(N=368) % | 4 mg/day
(N=364) % | All
Brexpiprazole (N=852) % |
||
| Gastrointestinal Disorders
|
|||||
| Dyspepsia
| 2
| 6
| 2
| 3
| 3
|
| Diarrhea
| 2
| 1
| 3
| 3
| 3
|
| Investigations
|
|||||
| Weight Increased
| 2
| 3
| 4
| 4
| 4
|
| Blood Creatinine Phosphokinase
Increased | 1
| 4
| 2
| 2
| 2
|
| Nervous System Disorders
|
|||||
| Akathisia
| 5
| 4
| 5
| 7
| 6
|
| Tremor
| 1
| 2
| 2
| 3
| 3
|
| Sedation
| 1
| 2
| 2
| 3
| 2
|
Extrapyramidal Symptoms
Adjunctive Treatment of Major Depressive Disorder
The incidence of reported extrapyramidal symptoms (EPS)-related adverse reactions, excluding akathisia, was 6% for brexpiprazole plus ADT-treated patients versus 3% for placebo plus ADT-treated patients. The incidence of akathisia events for brexpiprazole plus ADT-treated patients was 9% versus 2% for placebo plus ADT-treated patients.
In the 6-week placebo-controlled MDD studies, data was objectively collected on the Simpson-Angus Rating Scale (SAS) for EPS, the Barnes Akathisia Rating Scale (BARS) for akathisia and the Abnormal Involuntary Movement Score (AIMS) for dyskinesia. The mean change from baseline at last visit for brexpiprazole plus ADT-treated patients for the SAS, BARS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in brexpiprazole plus ADT-treated patients versus placebo plus ADT-treated patients for the BARS (4% versus 0.6%) and the SAS (4% versus 3%).
Schizophrenia
The incidence of reported EPS-related adverse reactions, excluding akathisia, was 5% for brexpiprazole-treated patients versus 4% for placebo-treated patients. The incidence of akathisia events for brexpiprazole-treated patients was 6% versus 5% for placebo-treated patients.
In the 6-week placebo-controlled, fixed-dose schizophrenia studies in adults, data was objectively collected on the Simpson-Angus Rating Scale (SAS) for EPS, the Barnes Akathisia Rating Scale (BARS) for akathisia and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia. The mean change from baseline at last visit for brexpiprazole-treated patients for the SAS, BARS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in brexpiprazole-treated patients versus placebo for the BARS (2% versus 1%) and the SAS (7% versus 5%).
Dystonia
Symptoms of dystonia may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Other Adverse Reactions Observed during Clinical Trial Evaluation of Brexpiprazole
Other adverse reactions (≥1% frequency and greater than placebo) within the short-term, placebo-controlled trials in adult patients with MDD and schizophrenia are shown below. The following listing does not include adverse reactions: 1) already listed in previous tables or elsewhere in the labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have clinically significant implications, or 5) which occurred at a rate equal to or less than placebo.
Eye Disorders:Vision Blurred
Gastrointestinal Disorders:Nausea, Dry Mouth, Salivary Hypersecretion, Abdominal Pain, Flatulence
Investigations:Blood Prolactin Increased
Musculoskeletal and Connective Tissue Disorders:Myalgia
Psychiatric Disorders:Abnormal Dreams
Skin and Subcutaneous Tissue Disorders:Hyperhidrosis
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti
®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of brexpiprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System disorders:Neuroleptic Malignant Syndrome
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Brexpiprazole
See Table 11 for clinically important drug interactions with brexpiprazole.
Table 11 Clinically Important Drug Interactions with Brexpiprazole
| Strong CYP3A4 Inhibitors
|
|
| Clinical Impact:
| Concomitant use of brexpiprazole with strong CYP3A4 inhibitors increased the exposure of brexpiprazole compared to the use of brexpiprazole alone
[see
Clinical Pharmacology (12.3)].
|
| Intervention:
| With concomitant use of brexpiprazole with a strong CYP3A4 inhibitor, reduce the brexpiprazole dosage
[see
Dosage and Administration (2.7)].
|
| Strong CYP2D6 Inhibitors*
|
|
| Clinical Impact:
| Concomitant use of brexpiprazole with strong CYP2D6 inhibitors increased the exposure of brexpiprazole compared to the use of brexpiprazole alone
[seeClinical Pharmacology (12.3)].
|
| Intervention:
| With concomitant use of brexpiprazole with a strong CYP2D6 inhibitor, reduce the brexpiprazole dosage
[see
Dosage and Administration (2.7)].
|
| Both CYP3A4 Inhibitors and CYP2D6 Inhibitors
|
|
| Clinical Impact:
| Concomitant use of brexpiprazole with 1) a strong CYP3A4 inhibitor and a
strong CYP2D6 inhibitor; or 2) a moderate CYP3A4 inhibitor and a strong CYP2D6 inhibitor; or 3) a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor; or 4) a moderate CYP3A4 inhibitor and a moderate CYP2D6 inhibitor increased the exposure of brexpiprazole compared to the use of brexpiprazole alone [see Clinical Pharmacology (12.3)]. |
| Intervention:
| With concomitant use of brexpiprazole with 1) a strong CYP3A4 inhibitor and a strong CYP2D6 inhibitor; or 2) a moderate CYP3A4 inhibitor and a strong CYP2D6 inhibitor; or 3) a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor; or 4) a moderate CYP3A4 inhibitor and a moderate CYP2D6 inhibitor, decrease the brexpiprazole dosage
[see
Dosage and Administration (2.7)].
|
| Strong CYP3A4 Inducers
|
|
| Clinical Impact:
| Concomitant use of brexpiprazole and a strong CYP3A4 inducer decreased the exposure of brexpiprazole compared to the use of brexpiprazole alone
[seeClinical Pharmacology (12.3)].
|
| Intervention:
| With concomitant use of brexpiprazole with a strong CYP3A4 inducer,
increase the brexpiprazole dosage [see Dosage and Administration (2.7)]. |
* In the clinical studies examining the adjunctive use of brexpiprazole in the treatment of MDD, dosage was not adjusted for strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine). Thus, CYP considerations are already factored into general dosing recommendations, and brexpiprazole may be administered without dosage adjustment in patients with MDD.
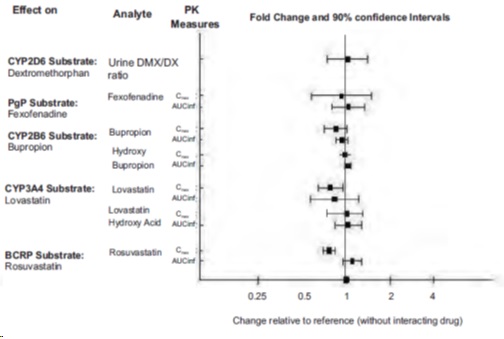
7.2 Drugs Having No Clinically Important Interactions with Brexpiprazole
Based on pharmacokinetic studies, no dosage adjustment of brexpiprazole is required when administered concomitantly with CYP2B6 inhibitors (e.g., ticlopidine) or gastric pH modifiers (e.g., omeprazole). Additionally, no dosage adjustment for substrates of CYP2D6 (e.g., dextromethorphan), CYP3A4 (e.g., lovastatin), CYP2B6 (e.g., bupropion), BCRP (e.g., rosuvastatin), or P-gp (e.g., fexofenadine) is required when administered concomitantly with brexpiprazole.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to brexpiprazole during pregnancy. For more information contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Adequate and well-controlled studies have not been conducted with brexpiprazole in pregnant women to inform drug-associated risks. However, neonates whose mothers are exposed to antipsychotic drugs, like brexpiprazole, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms. In animal reproduction studies, no teratogenicity was observed with oral administration of brexpiprazole to pregnant rats and rabbits during organogenesis at doses up to 73 and 146 times, respectively, of maximum recommended human dose (MRHD) of 4 mg/day on a mg/m
2basis. However, when pregnant rats were administered brexpiprazole during the period of organogenesis through lactation, the number of perinatal deaths of pups was increased at 73 times the MRHD
[see Data].The background risk of major birth defects and miscarriage for the indicated population(s) is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder, have been reported in neonates whose mothers were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately.
Data
Animal Data
Pregnant rats were treated with oral doses of 3, 10, and 30 mg/kg/day (7.3, 24, and 73 times the MRHD on a mg/m
2basis) of brexpiprazole during the period of organogenesis. Brexpiprazole was not teratogenic and did not cause adverse developmental effects at doses up to 73 times the MRHD.
Pregnant rabbits were treated with oral doses of 10, 30, and 150 mg/kg/day (49, 146, and 730 times the MRHD) of brexpiprazole during the period of organogenesis. Brexpiprazole was not teratogenic and did not cause adverse developmental effects at doses up to 146 times the MRHD. Findings of decreased body weight, retarded ossification, and increased incidences of visceral and skeletal variations were observed in fetuses at 730 times the MRHD, a dose that induced maternal toxicity.
In a study in which pregnant rats were administered oral doses of 3, 10, and 30 mg/kg/day (7.3, 24, and 73 times the MRHD) during the period of organogenesis and through lactation, the number of live-born pups was decreased, and early postnatal deaths increased at a dose 73 times the MRHD. Impaired nursing by dams, and low birth weight and decreased body weight gain in pups were observed at 73 times, but not at 24 times, the MRHD.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted to assess the presence of brexpiprazole in human milk, the effects of brexpiprazole on the breastfed infant, or the effects of brexpiprazole on milk production. Brexpiprazole is present in rat milk. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for brexpiprazole and any potential adverse effects on the breastfed infant from brexpiprazole or from the underlying maternal condition.
8.4 Pediatric Use
Major Depressive Disorder
Safety and effectiveness of brexpiprazole in pediatric patients with major depressive disorder have not been established. Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients [see
Boxed Warning, Warnings and Precautions (5.2)].
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti
®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Antipsychotic drugs increase the risk of death in elderly patients with dementia-related psychosis. Brexpiprazole is not approved for the treatment of patients with dementia-related psychosis
[see
Boxed Warning, Warnings and Precautions (5.1)].
Adjunctive Treatment of Major Depressive Disorder (MDD) and Schizophrenia
Of the total number of brexpiprazole-treated patients in the clinical studies for the adjunctive therapy to antidepressants for MDD and for schizophrenia, 248 (3%) were 65 years of age and older (which included 45 (18%) patients who were 75 years of age and older). Clinical studies of brexpiprazole in these patients did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients. In general, dosage selection for the treatment of MDD or schizophrenia in a geriatric patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, and cardiac function, concomitant diseases, and other drug therapy.
8.6 CYP2D6 Poor Metabolizers
Dosage adjustment is recommended in known CYP2D6 poor metabolizers because these patients have higher brexpiprazole concentrations than normal metabolizers of CYP2D6. Approximately 8% of Caucasians and 3 to 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The maximum recommended dosage in patients with moderate to severe hepatic impairment (Child-Pugh score ≥7) is lower than those with mild hepatic impairment and those with normal hepatic function [see Dosage and Administration (2.4)]. Patients with moderate to severe hepatic impairment generally had higher exposure to brexpiprazole than patients with normal hepatic function [see Clinical Pharmacology (12.3)]. Greater exposure may increase the risk of brexpiprazole-associated adverse reactions.
8.8 Renal Impairment
The maximum recommended dosage in patients with CrCl<60 mL/minute is lower than those with mild renal impairment and those with normal renal function [see Dosage and Administration (2.6)]. Patients with renal impairment had higher exposure to brexpiprazole than patients with normal renal function [see Clinical Pharmacology (12.3)]. Greater exposure may increase the risk of brexpiprazole-associated adverse reactions.
8.9 Other Specific Populations
The recommended dosage for brexpiprazole is the same in males and females, in different racial groups, and in smokers and nonsmokers [see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Brexpiprazole tablets contain brexpiprazole, which is not a controlled substance.
10 OVERDOSAGE
There is limited clinical trial experience regarding human overdosage with brexpiprazole.
Management of a brexpiprazole overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers. Consider contacting the Poison Help Line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
Oral activated charcoal and sorbitol (50 g/240 mL), administered one hour after ingesting oral brexpiprazole, decreased brexpiprazole C
maxand area under the curve (AUC) by approximately 5% to 23% and 31% to 39% respectively; however, there is insufficient information available on the therapeutic potential of activated charcoal in treating an overdose with brexpiprazole.
There is no information on the effect of hemodialysis in treating an overdose with brexpiprazole; hemodialysis is unlikely to be useful because brexpiprazole is highly bound to plasma proteins.
11 DESCRIPTION
Brexpiprazole, an atypical antipsychotic, is available as brexpiprazole tablets. Brexpiprazole is 7-[4-(4-(1-benzothiophen-4-yl) piperazin-1-yl) butoxy]-1, 2-dihydroquinolin-2-one. The molecular formula is C 25H 27N 3O 2S and its molecular weight is 433.57. The chemical structure is:
Brexpiprazole tablets are for oral administration and are available in 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg and 4 mg strengths. Inactive ingredients include corn starch, hypromellose, lactose monohydrate, low substituted hydroxypropyl cellulose, microcrystalline cellulose, sodium stearyl fumarate. The film coating contains FD&C Blue No. 1, FD&C Blue No. 2 and FD&C Red No. 40 (for 2 mg), FD&C Yellow No. 6 and FD&C Red No. 40 (for 3 mg), hypromellose, iron oxide black and iron oxide red (for 0.25 mg, 0.5 mg, and 1 mg), iron oxide yellow (for 0.25 mg and 1 mg), talc, titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of brexpiprazole in the adjunctive treatment of major depressive disorder, or treatment of schizophrenia is unknown. However, the efficacy of brexpiprazole may be mediated through a combination of partial agonist activity at serotonin 5-HT 1Aand dopamine D 2receptors, and antagonist activity at serotonin 5-HT 2Areceptors.
12.2 Pharmacodynamics
Brexpiprazole has affinity (expressed as K
i) for multiple monoaminergic receptors including serotonin 5-HT
1A(0.12 nM), 5-HT
2A(0.47 nM), 5-HT
2B(1.9 nM), 5-HT
7(3.7 nM), dopamine D
2(0.30 nM), D
3(1.1 nM), and noradrenergic α
1A(3.8 nM), α
1B(0.17 nM), α
1D(2.6 nM), and α
2C(0.59 nM) receptors. Brexpiprazole acts as a partial agonist at the 5-HT
1A, D
2, and D
3receptors and as an antagonist at 5-HT
2A, 5-HT
2B, 5-HT
7, α
1A, α
1B, α
1D, and α
2Creceptors. Brexpiprazole also exhibits affinity for histamine H
1receptor (19 nM) and for muscarinic M
1receptor (67% inhibition at 10 μM).
Cardiac Electrophysiology
At a dose 3 times the MRHD for the treatment of schizophrenia and 4 times the MRHD for adjunctive therapy to antidepressants for the treatment of MDD, brexpiprazole does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
After single-dose administration of brexpiprazole tablets, the peak plasma brexpiprazole concentrations occurred within 4 hours after administration, and the absolute oral bioavailability was 95%. Brexpiprazole steady-state concentrations were attained within 10 to 12 days of dosing.
Brexpiprazole can be administered with or without food. Administration of a 4 mg brexpiprazole tablet with a standard high-fat meal did not significantly affect the C
maxor AUC of brexpiprazole. After single and multiple once daily dose administration, brexpiprazole exposure (C
maxand AUC) increased in proportion to the dose administered.
In vitrostudies of brexpiprazole did not indicate that brexpiprazole is a substrate of efflux transporters such as MDRI (P-gp) and BCRP.
Distribution
The volume of distribution of brexpiprazole following intravenous administration is high (1.56 ± 0.42 L/kg), indicating extravascular distribution. Brexpiprazole is highly protein bound in plasma (greater than 99%) to serum albumin and α1-acid glycoprotein, and its protein binding is not affected by renal or hepatic impairment. Based on results of
in vitrostudies, brexpiprazole protein binding is not affected by warfarin, diazepam, or digitoxin.
Elimination
Metabolism
Based on
in vitrometabolism studies of brexpiprazole using recombinant human cytochrome P450 (CYP1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4), the metabolism of brexpiprazole was shown to be mainly mediated by CYP3A4 and CYP2D6.
In vivobrexpiprazole is metabolized primarily by CYP3A4 and CYP2D6 enzymes. After single- and multiple-dose administrations, brexpiprazole and its major metabolite, DM-3411, were the predominant drug moieties in the systemic circulation. At steady-state, DM-3411 represented 23% to 48% of brexpiprazole exposure (AUC) in plasma. DM-3411 is considered not to contribute to the therapeutic effects of brexpiprazole.
Based on
in vitrodata, brexpiprazole showed little to no inhibition of CYP450 isozymes.
Excretion
Following a single oral dose of [
14C]-labeled brexpiprazole, approximately 25% and 46% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 1% of unchanged brexpiprazole was excreted in the urine, and approximately 14% of the oral dose was recovered unchanged in the feces. Apparent oral clearance of a brexpiprazole oral tablet after once daily administration is 19.8 (±11.4) mL/h/kg. After multiple once-daily administrations of brexpiprazole, the terminal elimination half-lives of brexpiprazole and its major metabolite, DM-3411, were 91 hours and 86 hours, respectively.
Studies in Specific Populations
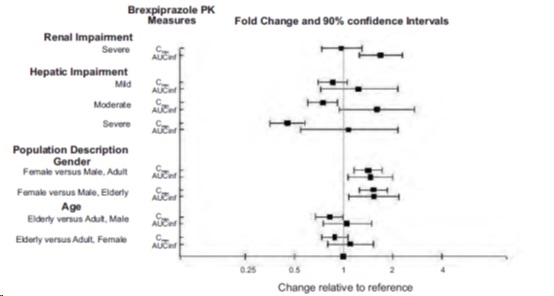
Exposure of brexpiprazole in specific populations are summarized in Figure 1. Population pharmacokinetic (PK) analysis indicated exposure of brexpiprazole in patients with moderate renal impairment was higher compared to patients with normal renal function.
Figure 1 Effect of Intrinsic Factors on Brexpiprazole Pharmacokinetics
Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti
®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, LTD.’S marketing exclusivity rights, this drug product is not labeled with that information.
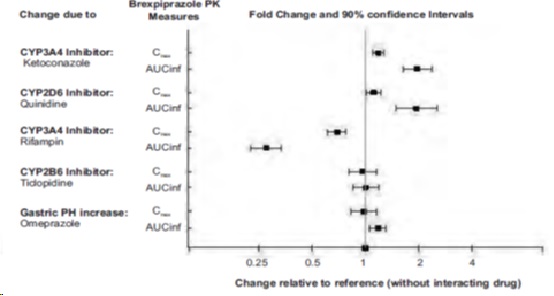
Drug Interaction Studies
Effect of other drugs on the exposures of brexpiprazole are summarized in Figure 2. Based on simulation, a 5.1-fold increase in AUC values at steady-state is expected when extensive metabolizers of CYP2D6 are administered with both strong CYP2D6 and CYP3A4 inhibitors. A 4.8-fold increase in mean AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors
[see
Drug Interactions (7.1)].
Figure 2 The Effect of Other Drugs on Brexpiprazole Pharmacokinetics
The effect of brexpiprazole on the exposures of other drugs are summarized in Figure 3.
Figure 3 The Effect of Brexpiprazole on Pharmacokinetics of Other Drugs
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in ICR mice and Sprague Dawley rats. Brexpiprazole was administered orally for two years to male and female mice at doses of 0.75, 2, and 5 mg/kg/day (0.9 to 6.1 times the oral MRHD of 4 mg/day based on mg/m
2body surface area) and to male and female rats at doses of 1, 3, and 10 mg/kg and 3, 10, and 30 mg/kg/day, respectively (2.4 to 24 and 7.3 to 73 times the oral MRHD, males and females). In female mice, the incidence of mammary gland adenocarcinoma was increased at all doses, and the incidence of adenosquamous carcinoma was increased at 2.4 and 6.1 times the MRHD. No increase in the incidence of tumors was observed in male mice. In the rat study, brexpiprazole was not carcinogenic in either sex at doses up to 73 times the MRHD.
Proliferative and/or neoplastic changes in the mammary and pituitary glands of rodents have been observed following chronic administration of antipsychotic drugs and are considered to be prolactin mediated. The potential for increasing serum prolactin level of brexpiprazole was shown in both mice and rats. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unknown.
Mutagenesis
Brexpiprazole was not mutagenic when tested in the
in vitrobacterial reverse mutation assay (Ames test). Brexpiprazole was negative for clastogenic activity in the
in vivomicronucleus assay in rats and was not genotoxic in the
in vivo/in vitrounscheduled DNA synthesis assay in rats.
In vitrowith mammalian cells brexpiprazole was clastogenic but only at doses that induced cytotoxicity. Based on a weight of evidence, brexpiprazole is not considered to present a genotoxic risk to humans.
Impairment of Fertility
Female rats were treated with oral doses of 0.3, 3, or 30 mg/kg/day (0.7, 7.3, and 73 times the oral MRHD on a mg/m
2basis) prior to mating with untreated males and continuing through conception and implantation. Estrus cycle irregularities and decreased fertility were observed at 3 and 30 mg/kg/day. Prolonged duration of pairing and increased preimplantation losses were observed at 30 mg/kg/day.
Male rats were treated with oral doses of 3, 10, or 100 mg/kg/day (7.3, 24, and 240 times the oral MRHD on a mg/m
2basis) for 63 days prior to mating with untreated females and throughout the 14 days of mating. No differences were observed in the duration of mating or fertility indices in males at any dose of brexpiprazole.
14 CLINICAL STUDIES
14.1 Adjunctive Treatment of Major Depressive Disorder
The efficacy of brexpiprazole in the adjunctive treatment of major depressive disorder (MDD) was evaluated in two 6-week double-blind, placebo-controlled, fixed-dose studies of adult patients meeting DSM-IV-TR criteria for MDD, with or without symptoms of anxiety, who had an inadequate response to prior antidepressant therapy (1 to 3 courses) in the current episode and who had also demonstrated an inadequate response throughout the 8 weeks of prospective antidepressant treatment (with escitalopram, fluoxetine, paroxetine controlled-release, sertraline, duloxetine delayed release, or venlafaxine extended release). Inadequate response during the prospective antidepressant treatment phase was defined as having persistent symptoms without substantial improvement throughout the course of treatment.
Patients in Study 1 (NCT01360645) were randomized to brexpiprazole 2 mg once a day or placebo. Patients in Study 2 (NCT01360632) were randomized to brexpiprazole 1 or 3 mg once a day or placebo. For patients randomized to brexpiprazole, all patients initiated treatment at 0.5 mg once daily during Week 1. At Week 2, the brexpiprazole dosage was increased to 1 mg in all treatment groups, and either maintained at 1 mg or increased to 2 mg or 3 mg once daily, based on treatment assignment, from Week 3 onwards. The dosages were then maintained for the 4 remaining weeks.
The primary endpoint was change from baseline to Week 6 in the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-related scale used to assess the degree of depressive symptomatology, with 0 representing no symptoms and 60 representing worst symptoms.
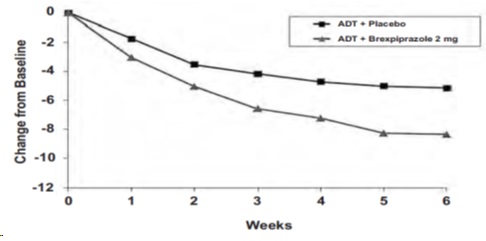
At randomization, the mean MADRS total score was 27. In Studies 1 and 2, brexpiprazole (plus ADT) 2 mg once daily and 3 mg once daily were superior to placebo plus ADT in reducing mean MADRS total scores. Results from the primary efficacy parameters for both fixed dose studies are shown below in Table 12. Figure 4 below shows the time course of response based on the primary efficacy measure (MADRS) in Study 1.
Table 12 Change in MADRS from Baseline at Week 6 in Adult Patients for Adjunctive Treatment of MDD (Study 1 and Study 2)
| Study
| Treatment Group
| N
| Mean Baseline
Score (SD) | LS Mean Change
from Baseline (SE) | Placebo-subtracted
Difference * (95% CI) |
| 1
| Brexpiprazole
(2 mg/day) + ADT † | 175
| 26.9 (5.7)
| -8.4 (0.6)
| -3.2 (-4.9, -1.5)
|
| Placebo + ADT
| 178
| 27.3 (5.6)
| -5.2 (0.6)
| --
|
|
| 2
| Brexpiprazole
(1 mg/day) + ADT | 211
| 26.5 (5.6)
| -7.6 (0.5)
| -1.3 (-2.7, 0.1)
|
| Brexpiprazole
(3 mg/day) + ADT | 213
| 26.5 (5.3)
| -8.3 (0.5)
| -2.0 (-3.4, -0.5)
|
|
| Placebo + ADT
| 203
| 26.5 (5.2)
| -6.3 (0.5)
| --
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval
* Difference (drug minus placebo) in least-squares mean change from baseline
†Dosages statistically significantly superior to placebo
An examination of population subgroups did not suggest differential response based on age, gender, race, or choice of prospective antidepressant.
Figure 4 Change from Baseline in MADRS Total Score by Study Visit (Week) in Patients with MDD in Adults (Study 1)
14.2 Schizophrenia
The efficacy of brexpiprazole in the treatment of adults with schizophrenia was demonstrated in two 6-week randomized, double-blind, placebo-controlled, fixed-dose clinical studies in patients who met DSM-IV-TR criteria for schizophrenia.
In both studies, Study 3 (NCT01396421) and Study 4 (NCT01393613), patients were randomized to brexpiprazole 2 or 4 mg once per day or placebo. Patients in the brexpiprazole groups initiated treatment at 1 mg once daily on Days 1 to 4. The brexpiprazole dosage was increased to 2 mg on Days 5 to 7. The dosage was then either maintained at 2 mg once daily or increased to 4 mg once daily, depending on treatment assignment, for the 5 remaining weeks.
The primary efficacy endpoint of both studies was the change from baseline to Week 6 in the Positive and Negative Syndrome Scale (PANSS) total score. The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 (best) to 210 (worst).
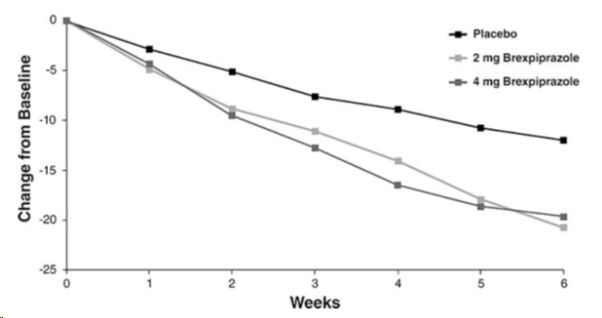
In Study 3, brexpiprazole at both 2 mg once daily and 4 mg once daily was superior to placebo on the PANSS total score. In Study 4, brexpiprazole 4 mg once daily was superior to placebo on the PANSS total score (Table 13).
Figure 5 shows the time course of response based on the primary efficacy measure (change from baseline in PANSS total score) in Study 3.
Examination of population subgroups based on age, sex, and race did not suggest differential responsiveness.
Table 13 Change in PANSS Total Score from Baseline at Week 6 in Adult Patients in Studies of Schizophrenia (Study 3 and Study 4)
| Study
| Treatment Group
| N
| Mean Baseline
Score (SD) | LS Mean Change
from Baseline (SE) | Placebo-subtracted
Difference * (95% CI) |
| 3
| Brexpiprazole
(2 mg/day) † | 180
| 95.9 (13.8)
| -20.7 (1.5)
| -8.7 (-13.1, -4.4)
|
| Brexpiprazole
(4 mg/day) † | 178
| 94.7 (12.1)
| -19.7 (1.5)
| -7.6 (-12.0, -3.1)
|
|
| Placebo
| 178
| 95.7 (11.5)
| -12.0 (1.6)
| --
|
|
| 4
| Brexpiprazole
(2 mg/day) | 179
| 96.3 (12.9)
| -16.6 (1.5)
| -3.1 (-7.2, 1.1)
|
| Brexpiprazole
(4 mg/day) † | 181
| 95.0 (12.4)
| -20.0 (1.5)
| -6.5 (-10.6, -2.4)
|
|
| Placebo
| 180
| 94.6 (12.8)
| -13.5 (1.5)
| --
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval
*Difference (drug minus placebo) in least-squares mean change from baseline
†Dosages statistically significantly superior to placebo
Figure 5 Change from Baseline in PANSS Total Score by Study Visit (Week) in Adult Patients with Schizophrenia (Study 3)
The safety and efficacy of brexpiprazole as maintenance treatment in adults with schizophrenia aged 18 to 65 years were demonstrated in the maintenance phase of a randomized withdrawal study (Study 5, NCT01668797). Patients were stabilized for at least 12 weeks on 1 to 4 mg/day of brexpiprazole (N=202). They were then randomized in the double-blind treatment phase to either continue brexpiprazole at their achieved stable dose (N=97), or to switch to placebo (N=105).
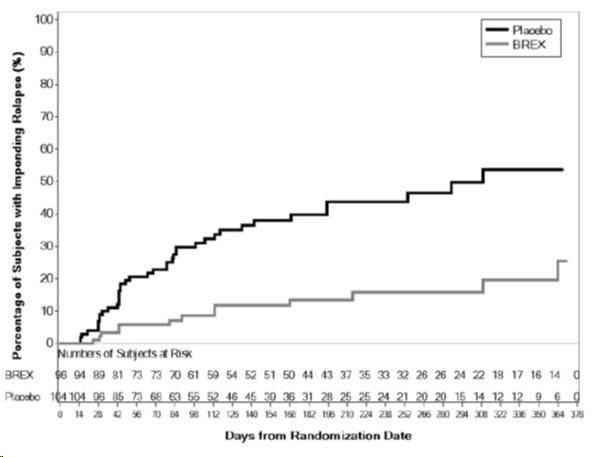
The primary endpoint in Study 5 was time from randomization to impending relapse during the double-blind phase, defined as: 1) Clinical Global Improvement score of ≥5 (minimally worse) and an increase to a score >4 on PANSS conceptual disorganization, hallucinatory behavior, suspiciousness, or unusual thought content items, with either a ≥2 increase on a specific item or ≥4 point increase on the combined four PANSS items, 2) hospitalization due to worsening of psychotic symptoms, 3) current suicidal behavior, or 4) violent/aggressive behavior.
A pre-specified interim analysis demonstrated a statistically significantly longer time to relapse in patients randomized to the brexpiprazole group compared to placebo-treated patients. The study was subsequently terminated early because maintenance of efficacy had been demonstrated. The Kaplan-Meier curves of the cumulative proportion of patients with relapse during the double-blind treatment phase for brexpiprazole and placebo groups are shown in Figure 6. The key secondary endpoint, the proportion of patients who met the criteria for impending relapse, was statistically significantly lower in brexpiprazole -treated patients compared with placebo group.
Figure 6 Kaplan-Meier Estimation of Percent Impending Relapse in Study 5
Note: A total of 202 patients were randomized. Among them, one patient in the placebo group did not take investigational medicinal product and one patient in the brexpiprazole group did not have post-randomization efficacy evaluations. These two patients were excluded from the efficacy analysis.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Brexpiprazole tablets have markings on both sides, and are available in the following strengths and package configurations (see below):
0.25 mg tablets are round shape grey colored film coated tablets with "B8" on one side and "H" on other side.
Bottle of 30 NDC: 31722-434-30
0.5 mg tablets are round shape pink colored film coated tablets with "B9" on one side and "H" on other side.
Bottle of 30 NDC: 31722-435-30
1 mg tablets are round shape beige colored film coated tablets with "B10" on one side and "H" on other side.
Bottle of 30 NDC: 31722-436-30
2 mg tablets are round shape blue colored film coated tablets with "B11" on one side and "H" on other side.
Bottle of 30 NDC: 31722-437-30
3 mg tablets are round shape red colored film coated tablets with "B12" on one side and "H" on other side.
Bottle of 30 NDC: 31722-438-30
4 mg tablets are round shape white to off white colored film coated tablets with "B13" on one side and "H" on other side.
Bottle of 30 NDC: 31722-439-30
Storage
Store brexpiprazole tablets at 20°C to 25°C (68°F to 77°F); excursions permitted to 15ºC to 30°C (59ºF to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling
(Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dosage is adjusted up or down, and instruct them to report such symptoms to the healthcare provider
[see
Boxed Warning, Warnings and Precautions (5.2)].
Dosage and Administration
Advise patients that brexpiprazole tablets can be taken with or without food. Advise patients regarding importance of following dosage escalation instructions
[see
Dosage and Administration (2)].
Neuroleptic Malignant Syndrome (NMS)
Counsel patients about a potentially fatal adverse reaction - neuroleptic malignant syndrome (NMS) - that has been reported in association with administration of antipsychotic drugs. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS
[see
Warnings and Precautions (5.4)].
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur
[see
Warnings and Precautions (5.5)].
Metabolic Changes
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight
[see
Warnings and Precautions (5.6)].
Pathological Gambling and Other Compulsive Behaviors
Advise patients and their caregivers of the possibility that they may experience compulsive urges to shop, intense urges to gamble, compulsive sexual urges, binge eating and/or other compulsive urges and the inability to control these urges while taking brexpiprazole tablets. In some cases, but not all, the urges were reported to have stopped when the dose was reduced or stopped
[see
Warnings and Precautions (5.7)].
Leukopenia, Neutropenia and Agranulocytosis
Advise patients with a pre-existing low WBC or a history of drug-induced leukopenia/neutropenia that they should have their CBC monitored while taking brexpiprazole tablets
[see
Warnings and Precautions (5.8)].
Orthostatic Hypotension and Syncope
Educate patients about the risk of orthostatic hypotension and syncope, especially early in treatment, and also at times of reinitiating treatment or increases in dosage
[see
Warnings and Precautions (5.9)].
Heat Exposure and Dehydration
Counsel patients regarding appropriate care in avoiding overheating and dehydration
[see
Warnings and Precautions (5.12)].
Potential for Cognitive and Motor Impairment
Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that brexpiprazole tablets therapy does not adversely affect their ability to engage in such activities
[see
Warnings and Precautions (5.14)].
Concomitant Medications
Advise patients to inform their healthcare providers of any changes to their current prescription or over-the-counter medications because there is a potential for clinically significant interactions
[see
Drug Interactions (7.1)].
Pregnancy
Advise patients that third trimester use of brexpiprazole tablets may cause extrapyramidal and/or withdrawal symptoms in a neonate and to notify their healthcare provider with a known or suspected pregnancy. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to brexpiprazole tablets during pregnancy
[see
Use in Specific Populations (8.1)].

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By:
HETEROTM
Hetero Labs Limited, Unit V, Polepally, Jadcherla,
Mahabubnagar - 509 301, India
Revised: 01/2024
MEDICATION GUIDE
| Brexpiprazole
(brex pip′ ra zole) tablets, for oral use |
|
What is the most important information I should know about brexpiprazole tablets? Brexpiprazole tablets may cause serious side effects, including: Increased risk of death in elderly people with dementia-related psychosis.Medicines like brexpiprazole tablets can raise the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia). Brexpiprazole tablets are not approved for the treatment of people with dementia-related psychosis. Increased risk of suicidal thoughts and actions.Brexpiprazole tablets and antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. o Depression and other mental illnesses are the most important causes of suicidal thoughts and actions. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member? o Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. This is very important when brexpiprazole tablets or the antidepressant medicine is started or when the dose is changed. o Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. o Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms. Call a healthcare provider right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What are brexpiprazole tablets? Brexpiprazole tablets are a prescription medicine used: along with antidepressant medicines to treat major depressive disorder (MDD) in adults to treat schizophrenia in adults It is not known if brexpiprazole tablets are safe and effective in children with MDD. It is not known if brexpiprazole tablets are safe and effective in children under 13 years of age with schizophrenia. |
|
Do not take brexpiprazole tablets if youare allergic to brexpiprazole or any of the ingredients in brexpiprazole tablets. See the end of this Medication Guide for a complete list of ingredients in brexpiprazole tablets. |
|
Before taking brexpiprazole tablets, tell your healthcare provider about all of your medical conditions, including if you: have or have had heart problems or a stroke have or have had low or high blood pressure have or have had diabetes or high blood sugar or a family history of diabetes or high blood sugar. Your healthcare provider should check your blood sugar before you start brexpiprazole tablets and during treatment with brexpiprazole tablets. have of have had high levels of total cholesterol, LDL cholesterol, or triglycerides, or low levels of HDL cholesterol have or have had seizures (convulsions) have or have had kidney or liver problems have or have had a low white blood cell count are pregnant or plan to become pregnant. Brexpiprazole tablets may harm your unborn baby. Taking brexpiprazole tablets during your third trimester of pregnancy may cause your baby to have abnormal muscle movements or withdrawal symptoms after birth. Talk to your healthcare provider about the risk to your unborn baby if you take brexpiprazole tablets during pregnancy. o Tell your healthcare provider if you become pregnant or think you are pregnant during treatment with brexpiprazole tablets. o There is a pregnancy exposure registry for women who are exposed to brexpiprazole tablets during pregnancy. If you become pregnant during treatment with brexpiprazole tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/. are breastfeeding or plan to breastfeed. It is not known if brexpiprazole passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with brexpiprazole tablets. Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Brexpiprazole tablets and other medicines may affect each other causing possible serious side effects. Brexpiprazole tablets may affect the way other medicines work, and other medicines may affect how brexpiprazole tablets work. Your healthcare provider can tell you if it is safe to take brexpiprazole tablets with your other medicines. Do not start or stop any medicines during treatment with brexpiprazole tablets without first talking to your healthcare provider. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
| How should I take brexpiprazole tablets?
Take brexpiprazole tablets exactly as your healthcare provider tells you to take it. Your healthcare provider may change your dose if needed. Do not change the dose or stop taking brexpiprazole tablets without first talking to your healthcare provider. Take brexpiprazole tablets 1 time each day with or without food. If you take too much brexpiprazole, call your healthcare provider or Poison Help Line at 1-800-222-1222 or go to the nearest hospital emergency room right away. |
| What should I avoid while taking brexpiprazole tablets?
Do not drive a car, operate machinery, or do other dangerous activities until you know how brexpiprazole tablets affects you. Brexpiprazole tablets may make you feel drowsy. Do not become too hot or dehydrated during treatment with brexpiprazole tablets. o Do not exercise too much. o In hot weather, stay inside in a cool place if possible. o Stay out of the sun. o Do not wear too much clothing or heavy clothing. o Drink plenty of water. |
| What are the possible side effects of brexpiprazole tablets?
Brexpiprazole tablets may cause serious side effects, including: See “What is the most important information I should know about brexpiprazole tablets?” Cerebrovascular problems, including stroke, in elderly people with dementia-related psychosis that can lead to death. Neuroleptic malignant syndrome (NMS) is a serious condition that can lead to death. Call your healthcare provider or go to the nearest hospital emergency room right away if you have some or all of the following signs and symptoms of NMS: o high fever o changes in your pulse, blood pressure, heart rate, and breathing o stiff muscles o increased sweating o confusion\ Uncontrolled body movements (tardive dyskinesia).Brexpiprazole tablets may cause movements that you cannot control in your face, tongue, or other body parts. Tardive dyskinesia may not go away, even if you stop taking brexpiprazole tablets. Tardive dyskinesia may also start after you stop taking brexpiprazole tablets. Problems with your metabolism such as: o high blood sugar (hyperglycemia) and diabetes.Increases in blood sugar can happen in some people who take brexpiprazole tablets. Extremely high blood sugar can lead to coma or death. Your healthcare provider should check your blood sugar before you start, or soon after you start brexpiprazole tablets and then regularly during long term treatment with brexpiprazole tablets. Call your healthcare provider if you have any of these symptoms of high blood sugar during treatment with brexpiprazole tablets: feel very thirsty need to urinate more than usual feel very hungry feel weak or tired feel sick to your stomach feel confused, or your breath smells fruity o increased fat levels (cholesterol and triglycerides) in your blood.Your healthcare provider should check the fat levels in your blood before you start, or soon after you start brexpiprazole tablets, and then periodically during treatment with brexpiprazole tablets. o weight gain.You and your healthcare provider should check your weight before you start and often during treatment with brexpiprazole tablets. Unusual and uncontrollable (compulsive) urges.Some people taking brexpiprazole tablets have had strong unusual urges, to gamble and gambling that cannot be controlled (compulsive gambling). Other compulsive urges include sexual urges, shopping, and eating or binge eating. If you or your family members notice that you are having new or unusual strong urges or behaviors, talk to your healthcare provider. Low white blood cell count.Your healthcare provider may do blood tests during the first few months of treatment with brexpiprazole tablets. Decreased blood pressure (orthostatic hypotension) and fainting.You may feel dizzy, lightheaded, or pass out (faint) when you rise too quickly from a sitting or lying position. Falls.Brexpiprazole tablets may make you sleepy or dizzy, may cause a decrease in your blood pressure when changing position (orthostatic hypotension), and can slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries. Seizures (convulsions). Problems controlling your body temperature so that you feel too warm. See “What should I avoid while taking brexpiprazole tablets?” Difficulty swallowing that can cause food or liquid to get into your lungs. Sleepiness, drowsiness, feeling tired, difficulty thinking and doing normal activities.See “What should I avoid while taking brexpiprazole tablets?” The most common side effects of brexpiprazole tabletsinclude weight gain, sleepiness, dizziness, common cold symptoms, and restlessness or feeling like you need to move (akathisia). These are not all the possible side effects of brexpiprazole tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| How should I store brexpiprazole tablets?
Store brexpiprazole tablets at room temperature between 68°F to 77°F (20°C to 25°C). Keep brexpiprazole tablets and all medicines out of the reach of children. |
| General information about the safe and effective use of brexpiprazole tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use brexpiprazole tablets for a condition for which it was not prescribed. Do not give brexpiprazole tablets to other people, even if they have the same symptoms you have. They may harm them. You can ask your pharmacist or healthcare provider for information about brexpiprazole tablets that is written for health professionals. |
| What are the ingredients in brexpiprazole tablets?
Active ingredient:brexpiprazole Inactive ingredients:corn starch, hypromellose, lactose monohydrate, low substituted hydroxypropyl cellulose, microcrystalline cellulose, sodium stearyl fumarate. The film coating contains FD&C Blue No. 1, FD&C Blue No. 2 and FD&C Red No. 40 (for 2 mg), FD&C Yellow No. 6 and FD&C Red No. 40 (for 3 mg), hypromellose, iron oxide black and iron oxide red (for 0.25 mg, 0.5 mg, and 1 mg), iron oxide yellow (for 0.25 mg and 1 mg), talc, titanium dioxide. Pediatric use information is approved for Otsuka Pharmaceutical Company, Ltd.’s Rexulti ®(brexpiprazole) tablets. However, due to Otsuka Pharmaceutical Company, Ltd.’s marketing exclusivity rights, this drug product is not labeled with that information. 
Manufactured for: Camber Pharmaceuticals, Inc. Piscataway, NJ 08854 By: HETEROTM Hetero Labs Limited, Unit V, Polepally, Jadcherla, Mahabubnagar - 509 301, India For more information about brexpriprazole tablets, call 1-866-495-1995. |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 01/2024
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Brexpiprazole Tablets Container Label - 0.25 mg
Brexpiprazole Tablets Container Carton - 0.25 mg

Brexpiprazole Tablets Container Label - 0.5 mg
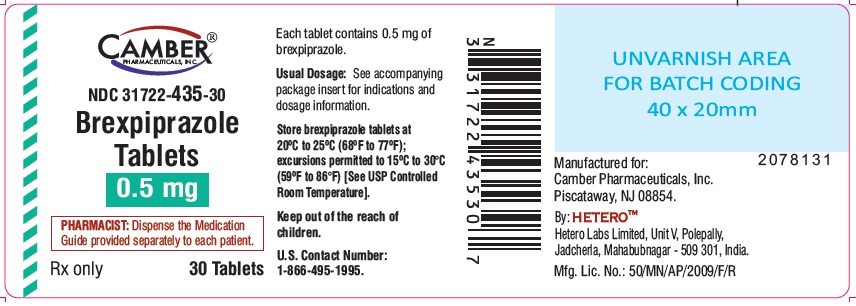
Brexpiprazole Tablets Container Carton - 0.5 mg

Brexpiprazole Tablets Container Label – 1 mg

Brexpiprazole Tablets Container Carton – 1 mg

Brexpiprazole Tablets Container Label – 2 mg
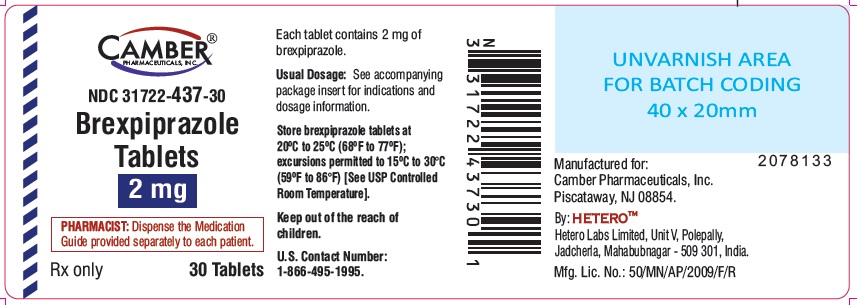
Brexpiprazole Tablets Container Carton – 2 mg

Brexpiprazole Tablets Container Label – 3 mg
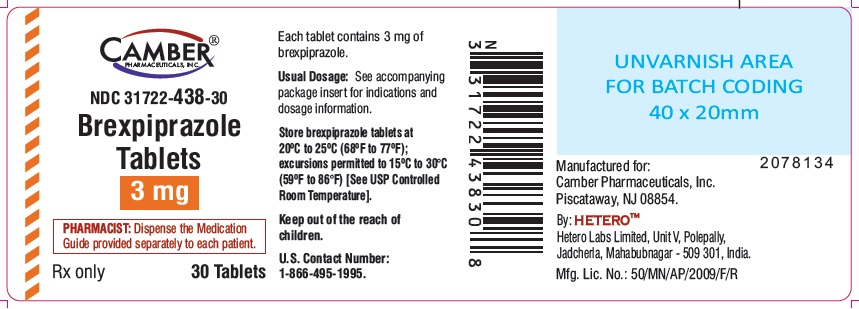
Brexpiprazole Tablets Container Carton – 3 mg

Brexpiprazole Tablets Container Label – 4 mg
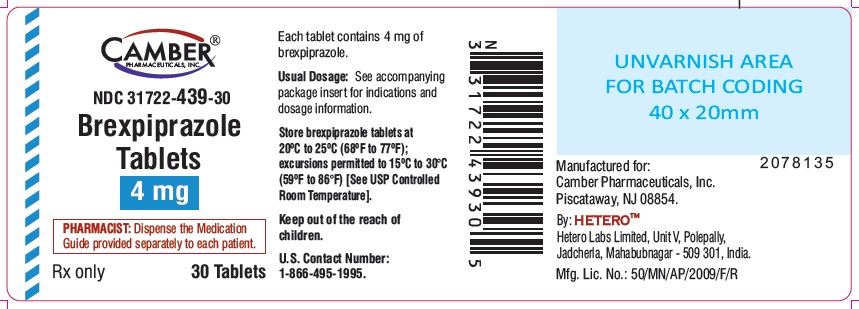
Brexpiprazole Tablets Container Carton – 4 mg

| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| BREXPIPRAZOLE
brexpiprazole tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Camber Pharmaceuticals, Inc. (826774775) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hetero Labs Limited Unit V | 650452530 | manufacture(31722-434, 31722-435, 31722-436, 31722-437, 31722-438, 31722-439) | |