Guaifenesin by Apace Packaging, LLC / Advance Pharmaceutical Inc GUAIFENESIN tablet
Guaifenesin by
Drug Labeling and Warnings
Guaifenesin by is a Otc medication manufactured, distributed, or labeled by Apace Packaging, LLC, Advance Pharmaceutical Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredient
- Purpose
- Uses
-
WARNINGS
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm(mucus)
stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash or persistent headache. This could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Manufactured for: AvKARE, Inc., Pulaski, TN 38478 Mfg. Rev. 04/15 AV 09/16 (P)
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 50268-382-15
Guaifenesin Tablets USP
200mg
EXPECTORANT
For Ages 12 and Over
50 Tablets (5 x 10) Unit Dose
5026838215
NDC: 50268-382-15
Guaifenesin Tablets USP
200mg
EXPECTORANT
For Ages 12 and Over
50 Tablets (5 x 10) Unit Dose
5026838215Drug Facts
Active ingredient Purpose
(in each immediate-release tablet)
Guaifenesin USP, 200 mg ...................... Expectorant
Uses helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive.
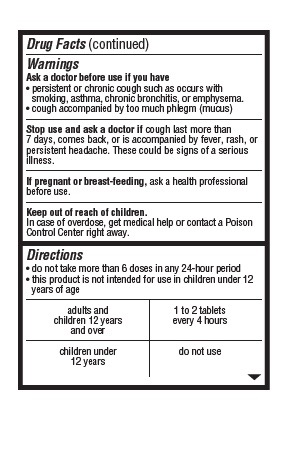
Drug Facts (continued)
Warnings
Ask a doctor before use if you have
persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if cough last more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
do not take more than 6 doses in any 24-hour period
this product is not intended for use in children under 12 years of age
adults and children 12 years and over 1 to 2 tablets every 4 hours
children under 12 years do not use
Drug Facts (continued)
Other information
store at 15° to 30°C (59° to 86°F).
You may report serious side effects to: AvKARE, Inc. at 1 (855) 361-3993
Inactive ingredients
colloidal silicon dioxide, FD&C red #40 (Al-lake), magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, stearic acid.Manufactured for: AvKARE, Inc., Pulaski, TN 38478
Mfg. Rev. 04/15 AV 09/16 (P)

-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 15338-769(NDC: 0603-4890) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color red Score score with uneven pieces Shape ROUND Size 10mm Flavor Imprint Code AP;151 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15338-769-05 50 in 1 BOX, UNIT-DOSE 10/04/2016 1 NDC: 15338-769-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/04/2016 Labeler - Apace Packaging, LLC (361961142) Establishment Name Address ID/FEI Business Operations Advance Pharmaceutical Inc 078301063 manufacture(15338-769) Establishment Name Address ID/FEI Business Operations Apace Packaging, LLC 361961142 repack(15338-769) , label(15338-769)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.