MULTI-SYMPTOM DAYTIME AND NIGHTTIME COLD AND FLU RELIEF PLUS- acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl kit
Multi-Symptom Daytime and Nighttime Cold and Flu Relief Plus by
Drug Labeling and Warnings
Multi-Symptom Daytime and Nighttime Cold and Flu Relief Plus by is a Otc medication manufactured, distributed, or labeled by Walgreens Company, Apotex Inc., Accucaps Industries Limited, Accucaps Industries Limited Strathroy, Legacy Pharmaceutical Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients for DAYTIME ONLY (in each LiquidCap)
- Active ingredients for NIGHTTIME (in each LiquidCap)
- Purpose (DAYTIME ONLY)
- Purpose (NIGHTTIME ONLY)
- Uses (DAYTIME ONLY)
- Uses (NIGHTTIME ONLY)
-
Warnings (DAYTIME ONLY)
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 10 capsules in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- cough with excessive phlegm (mucus)
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
-
Warnings (NIGHTTIME ONLY)
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 10 capsules in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use to sedate children.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough with excessive phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- Directions (DAYTIME ONLY)
- Directions (NIGHTTIME)
- Other Information
- Inactive ingredients (DAYTIME ONLY)
- Inactive ingredients (NIGHTTIME)
- Questions or comments?
-
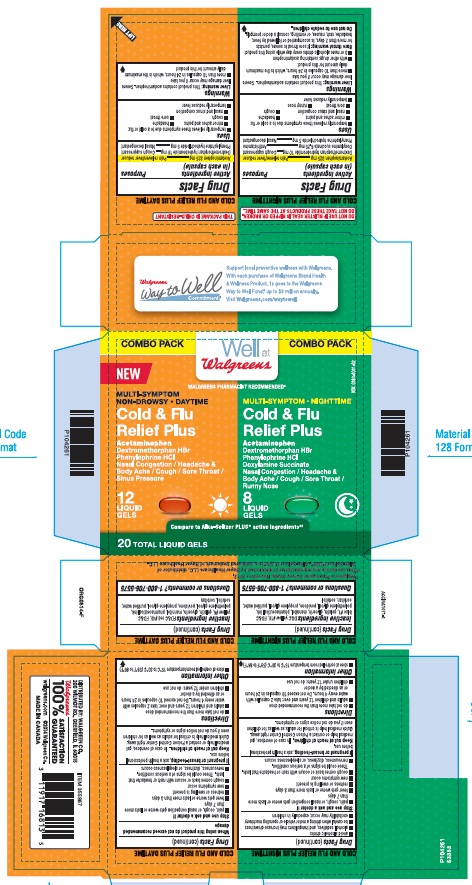
Principal Display Panel
Combo Pack
MULTI-SYMPTOM NON-DROWSY DAYTIME
Cold & Flu Relief Plus
Acetaminophen
Aches/Fever/Sore Throat
Dextromethorphan HBr/Cough
Phenylephrine HCl/Nasal Congestion
12 LIQUID GELS
MULTI-SYMPTOM NIGHTTIME
Cold & Flu Relief Plus
Aches/Fever/Sore Throat
Dextromethorphan HBr/Cough
Doxylamine Succinate/Sneezing/Runny Nose
8 LIQUID CAPS
Compare to Alka Seltzer PLUS® active ingredients‡‡
20 TOTAL LIQUID GELS
-
INGREDIENTS AND APPEARANCE
MULTI-SYMPTOM DAYTIME AND NIGHTTIME COLD AND FLU RELIEF PLUS
acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-1031 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-1031-02 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 12 Part 2 1 BLISTER PACK 8 Part 1 of 2 MULTI-SYMPTOM DAY TIME COLD AND FLU RELIEF PLUS
acetaminophen, dextromethorphan hbr, phenylephrine hcl capsule, liquid filledProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MANNITOL (UNII: 3OWL53L36A) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color ORANGE Score no score Shape OVAL Size 20mm Flavor Imprint Code 95A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/01/2013 Part 2 of 2 MULTI-SYMPTOM NIGHT TIME COLD AND FLU RELIEF PLUS
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate capsule, liquid filledProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONES (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color GREEN Score no score Shape OVAL Size 20mm Flavor Imprint Code 35A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/01/2014 Labeler - Walgreens Company (008965063) Registrant - Apotex Inc. (209429182) Establishment Name Address ID/FEI Business Operations Accucaps Industries Limited 248441727 analysis(0363-1031) , manufacture(0363-1031) Establishment Name Address ID/FEI Business Operations Accucaps Industries Limited Strathroy 243944050 analysis(0363-1031) , manufacture(0363-1031) Establishment Name Address ID/FEI Business Operations Legacy Pharmaceutical Packaging 969852743 pack(0363-1031)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.