LITHIUM CARBONATE capsule
Lithium Carbonate by
Drug Labeling and Warnings
Lithium Carbonate by is a Prescription medication manufactured, distributed, or labeled by Hetero Drugs Limited, Hetero Labs Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
WARNING
Lithium toxicity is closely related to serum lithium levels, and can occur at doses close to therapeutic levels. Facilities for prompt and accurate serum lithium determinations should be available before initiating therapy (see DOSAGE AND ADMINISTRATION). -
DESCRIPTION
Each capsule for oral administration contains: Lithium Carbonate USP……… 150 mg, 300 mg and 600 mg.
Inactive Ingredients
The capsules contain talc. The hard gelatin shell consists of gelatin, titanium dioxide, sodium lauryl sulphate and FD & C Red 40.
The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide E172 dye, potassium hydroxide.
Lithium is an element of the alkali-metal group with atomic number 3, atomic weight 6.94, and an emission line at 671 nm on the flame photometer.
The empirical formula for Lithium Citrate is C6H 5Li3O7; molecular weight 209.92. Lithium acts as an antimanic.
Lithium Carbonate is a white, light, alkaline powder with molecular formula Li2CO3 and molecular weight 73.89. - CLINICAL PHARMACOLOGY
-
INDICATIONS & USAGE
Lithium Carbonate Capsule USP is indicated in the treatment of manic episodes of Bipolar Disorder. Bipolar Disorder, Manic (DSM-III) is equivalent to Manic Depressive illness, Manic, in the older DSM-II terminology.
Lithium Carbonate Capsule USP is also indicated as a maintenance treatment for individuals with a diagnosis of Bipolar Disorder. Maintenance therapy reduces the frequency of manic episodes and diminishes the intensity of those episodes which may occur.
Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, elation, poor judgment, aggressiveness, and possibly hostility. When given to a patient experiencing a manic episode, lithium may produce a normalization of symptomatology within 1 to 3 weeks. -
CONTRAINDICATIONS
Lithium should generally not be given to patients with significant renal or cardiovascular disease, severe debilitation or dehydration, or sodium depletion, and to patients receiving diuretics, since the risk of lithium toxicity is very high in such patients. If the psychiatric indication is life-threatening, and if such a patient fails to respond to other measures, lithium treatment may be undertaken with extreme caution, including daily serum lithium determinations and adjustment to the usually low doses ordinarily tolerated by these individuals. In such instances, hospitalization is a necessity. -
WARNINGS
Lithium toxicity is closely related to serum lithium levels, and can occur at doses close to therapeutic levels (see DOSAGE AND ADMINISTRATION).Unmasking of Brugada Syndrome
There have been postmarketing reports of a possible association between treatment with lithium and the unmasking of Brugada Syndrome. Brugada Syndrome is a disorder characterized by abnormal electrocardiographic (ECG) findings and a risk of sudden death. Lithium should generally be avoided in patients with Brugada Syndrome or those suspected of having Brugada Syndrome. Consultation with a cardiologist is recommended if: (1) treatment with lithium is under consideration for patients suspected of having Brugada Syndrome or patients who have risk factors for Brugada Syndrome, e.g., unexplained syncope, a family history of Brugada Syndrome, or a family history of sudden unexplained death before the age of 45 years, (2) patients who develop unexplained syncope or palpitations after starting lithium therapy.Pregnancy
Lithium may cause fetal harm when administered to a pregnant woman. There have been reports of lithium having adverse effects on nidations in rats, embryo viability in mice, and metabolism in-vitro of rat testis and human spermatozoa have been attributed to lithium, as have teratogenicity in submammalian species and cleft palates in mice. Studies in rats, rabbits and monkeys have shown no evidence of lithium-induced teratology. Data from lithium birth registries suggest an increase in cardiac and other anomalies, especially Ebstein’s anomaly. If the patient becomes pregnant while taking lithium, she should be apprised of the potential risk to the fetus. If possible, lithium should be withdrawn for at least the first trimester unless it is determined that this would seriously endanger the mother.Lithium Induced Renal Effects
Chronic lithium therapy may be associated with diminution of renal concentrating ability, occasionally presenting as nephrogenic diabetes insipidus, with polyuria and polydipsia. Such patients should be carefully managed to avoid dehydration with resulting lithium retention and toxicity. This condition is usually reversible when lithium is discontinued.
Morphologic changes with glomerular and interstitial fibrosis and nephron-atrophy have been reported in patients on chronic lithium therapy. Morphologic changes have also been seen in bipolar patients never exposed to lithium. The relationship between renal functional and morphologic changes and their association with lithium therapy has not been established.
When kidney function is assessed, for baseline data prior to starting lithium therapy or thereafter, routine urinalysis and other tests may be used to evaluate tubular function (e.g., urine specific gravity or osmolality following a period of water deprivation, or 24-hour urine volume) and glomerular function (e.g., serum creatinine or creatinine clearance). During lithium therapy, progressive or sudden changes in renal function, even within the normal range, indicate the need for reevaluation of treatment. -
PRECAUTIONS
General
The ability to tolerate lithium is greater during the acute manic phase and decreases when manic symptoms subside (see DOSAGE AND ADMINISTRATION).
The distribution space of lithium approximates that of total body water. Lithium is primarily excreted in urine with insignificant excretion in feces. Renal excretion of lithium is proportional to its plasma concentration. The half-life of elimination of lithium is approximately 24 hours. Lithium decreases sodium reabsorption by the renal tubules which could lead to sodium depletion. Therefore, it is essential for the patient to maintain a normal diet, including salt, and an adequate fluid intake (2500 to 3000 mL) at least during the initial stabilization period. Decreased tolerance to lithium has been reported to ensue from protracted sweating or diarrhea and, if such occur, supplemental fluid and salt should be administered.
In addition to sweating and diarrhea, concomitant infection with elevated temperatures may also necessitate a temporary reduction or cessation of medication.
Previously existing underlying thyroid disorders do not necessarily constitute a contraindication to lithium treatment; where hypothyroidism exists, careful monitoring of thyroid function during lithium stabilization and maintenance allows for correction of changing thyroid parameters, if any. Where hypothyroidism occurs during lithium stabilization and maintenance, supplemental thyroid treatment may be used.Information for Patients
A condition known as Brugada Syndrome may pre-exist and be unmasked by lithium therapy. Brugada Syndrome is a heart disorder characterized by abnormal electrocardiographic (ECG) findings and risk of sudden death. Patients should be advised to seek immediate emergency assistance if they experience fainting, lightheadedness, abnormal heart beats, or shortness of breath.
Outpatients and their families should be warned that the patient must discontinue lithium therapy and contact his physician if such clinical signs of lithium toxicity as diarrhea, vomiting, tremor, mild ataxia, drowsiness, or muscular weakness occur.
Lithium may impair mental and/or physical abilities. Caution patients about activities requiring alertness (e.g., operating vehicles or machinery).Drug Interactions
Combined Use Of Haloperidol and Lithium.
An encephalopathic syndrome (characterized by weakness, lethargy, fever, tremulousness and confusion, extrapyramidal symptoms, leucocytosis, elevated serum enzymes, BUN and FBS) followed by irreversible brain damage has occurred in a few patients treated with lithium plus haloperidol. A causal relationship between these events and the concomitant administration of lithium and haloperidol has not been established; however, patients receiving such combined therapy should be monitored closely for early evidence of neurological toxicity and treatment discontinued promptly if such signs appear.
The possibility of similar adverse interactions with other antipsychotic medication exists.
Lithium may prolong the effects of neuromuscular blocking agents. Therefore, neuromuscular blocking agents should be given with caution to patients receiving lithium.
Caution should be used when lithium and diuretics or angiotensin converting enzyme (ACE) inhibitors are used concomitantly because sodium loss may reduce the renal clearance of lithium and increase serum lithium levels with risk of lithium toxicity. When such combinations are used, the lithium dosage may need to be decreased, and more frequent monitoring of lithium plasma levels is recommended.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDS):
Lithium levels should be closely monitored when patients initiate or discontinue NSAID use. In some cases, lithium toxicity has resulted from interactions between an NSAID and lithium. Indomethacin and piroxicam have been reported to increase significantly steady-state plasma lithium concentrations. There is also evidence that other nonsteroidal anti-inflammatory agents, including the selective cyclooxygenase-2 (COX-2) inhibitors, have the same effect. In a study conducted in healthy subjects, mean steady-state lithium plasma levels increased approximately 17% in subjects receiving lithium 450 mg BID with celecoxib 200 mg BID as compared to subjects receiving lithium alone.Nursing Mothers
Lithium is excreted in human milk. Nursing should not be undertaken during lithium therapy except in rare and unusual circumstances where, in the view of the physician, the potential benefits to the mother outweigh possible hazards to the child.Usage in Children
Since information regarding the safety and effectiveness of lithium in children under 12 years of age is not available, its use in such patients is not recommended at this time. There has been a report of a transient syndrome of acute dystonia and hyperreflexia occurring in a 15 kg child who ingested 300 mg of lithium carbonate.
-
ADVERSE REACTIONS
Lithium Toxicity
The likelihood of toxicity increases with increasing serum lithium levels. Serum lithium levels greater than 1.5 mEq/mL carry a greater risk than lower levels. However, patients sensitive to lithium may exhibit toxic signs at serum levels below 1.5 mEq/mL.
Diarrhea, vomiting, drowsiness, muscular weakness and lack of coordination may be early signs of lithium toxicity, and can occur at lithium levels below 2.0 mEq/mL. At higher levels, giddiness, ataxia, blurred vision, tinnitus and a large output of dilute urine may be seen. Serum lithium levels above 3.0 mEq/mL may produce a complex clinical picture involving multiple organs and organ systems. Serum lithium levels should not be permitted to exceed 2.0 mEq/mL during the acute treatment phase.
Fine hand tremor, polyuria and mild thirst may occur during initial therapy for the acute manic phase, and may persist throughout treatment. Transient and mild nausea and general discomfort may also appear during the first few days of lithium administration.
These side effects are an inconvenience rather than a disabling condition, and usually subside with continued treatment or a temporary reduction or cessation of dosage. If persistent, a cessation of dosage is indicated.
The following adverse reactions have been reported and do not appear to be directly related to serum lithium levels.
Neuromuscular:
Tremor, muscle hyperirritability (fasciculations, twitching, clonic movements of whole limbs), ataxia, choreo-athetotic movements, hyperactive deep tendon reflexes.
Central Nervous System:
Blackout spells, epileptiform seizures, slurred speech, dizziness, vertigo, incontinence of urine or feces, somnolence, psychomotor retardation, restlessness, confusion, stupor, coma, acute dystonia, downbeat nystagmus.
Cardiovascular:
Cardiac arrhythmia, hypotension, peripheral circulatory collapse, sinus node dysfunction with severe bradycardia (which may result in syncope), unmasking of Brugada Syndrome (See WARNINGS: Unmasking of Brugada Syndromeand PRECAUTIONS: Information for the Patients).
Neurological:
Cases of pseudotumor cerebri (increased intracranial pressure and papilledema) have been reported with lithium use. If undetected, this condition may result in enlargement of the blind spot, constriction of visual fields and eventual blindness due to optic atrophy. Lithium should be discontinued, if clinically possible, if this syndrome occurs.
Gastrointestinal:
Anorexia, nausea, vomiting, diarrhea.
Genitourinary:
Albuminuria, oliguria, polyuria, glycosuria.
Dermatologic:
Drying and thinning of hair, anesthesia of skin, chronic folliculitis, xerosis cutis, alopecia and exacerbation of psoriasis.
Autonomic Nervous System:
Blurred vision, dry mouth.
Thyroid Abnormalities:
Euthyroid goiter and/or hypothyroidism (including myxedema) accompanied by lower T3 and T4. Iodine 131 uptake may be elevated. (See PRECAUTIONS). Paradoxically, rare cases of hyperthyroidism have been reported.
EEG Changes:
Diffuse slowing, widening of frequency spectrum, potentiation and disorganization of background rhythm.
EKG Changes:
Reversible flattening, isoelectricity or inversion of T-waves.
Miscellaneous:
Fatigue, lethargy, transient scotomata, dehydration, weight loss, tendency to sleep.
Miscellaneous Reactions Unrelated to Dosage are:
Transient electroencephalographic and electrocardiographic changes, leucocytosis, headache, diffuse non-toxic goiter with or without hypothyroidism, transient hyperglycemia, generalized pruritis with or without rash, cutaneous ulcers, albuminuria, worsening of organic brain syndromes, excessive weight gain, edematous swelling of ankles or wrists, and thirst or polyuria, sometimes resembling diabetes insipidus, and metallic taste.
A single report has been received of the development of painful discoloration of fingers and toes and coldness of the extremities within one day of the starting of treatment of lithium. The mechanism through which these symptoms (resembling Raynaud’s Syndrome) developed is not known. Recovery followed discontinuance. -
OVERDOSAGE
The toxic levels for lithium are close to the therapeutic levels. It is therefore important that patients and their families be cautioned to watch for early symptoms and to discontinue the drug and inform the physician should they occur. Toxic symptoms are listed in detail under ADVERSE REACTIONS.
Treatment
No specific antidote for lithium poisoning is known. Early symptoms of lithium toxicity can usually be treated by reduction of cessation of dosage of the drug and resumption of the treatment at a lower dose after 24 to 48 hours. In severe cases of lithium poisoning, the first and foremost goal of treatment consists of elimination of this ion from the patient.
Treatment is essentially the same as that used in barbiturate poisoning: 1) gastric lavage, 2) correction of fluid and electrolyte imbalance and 3) regulation of kidney functioning. Urea, mannitol, and aminophylline all produce significant increases in lithium excretion. Hemodialysis is an effective and rapid means of removing the ion from the severely toxic patient. Infection prophylaxis, regular chest X-rays, and preservation of adequate respiration are essential. -
DOSAGE & ADMINISTRATION
Acute Mania
Optimal patient response to Lithium Carbonate usually can be established and maintained with 600 mg t.i.d. Such doses will normally produce an effective serum lithium level ranging between 1.0 and 1.5 mEq/L. Dosage must be individualized according to serum levels and clinical response. Regular monitoring of the patient’s clinical state and of serum lithium levels is necessary. Serum levels should be determined twice per week during the acute phase, and until the serum level and clinical condition of the patient have been stabilized.
Long-Term Control
The desirable serum lithium levels are 0.6 to 1.2 mEq/mL. Dosage will vary from one individual to another, but usually 300 mg of Lithium Carbonate t.i.d. or q.i.d., will maintain this level. Serum lithium levels in uncomplicated cases receiving maintenance therapy during remission should be monitored at least every two months.
Patients abnormally sensitive to lithium may exhibit toxic signs at serum levels of 1.0 to 1.5 mEq/mL. Elderly patients often respond to reduced dosage, and may exhibit signs of toxicity at serum levels ordinarily tolerated by other patients.
N.B.
Blood samples for serum lithium determination should be drawn immediately prior to the next dose when lithium concentrations are relatively stable (i.e., 8 to 12 hours after the previous dose). Total reliance must not be placed on serum levels alone. Accurate patient evaluation requires both clinical and laboratory analysis. -
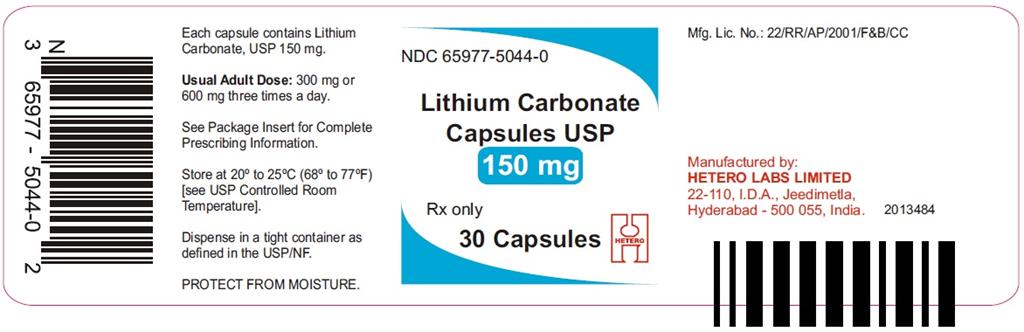
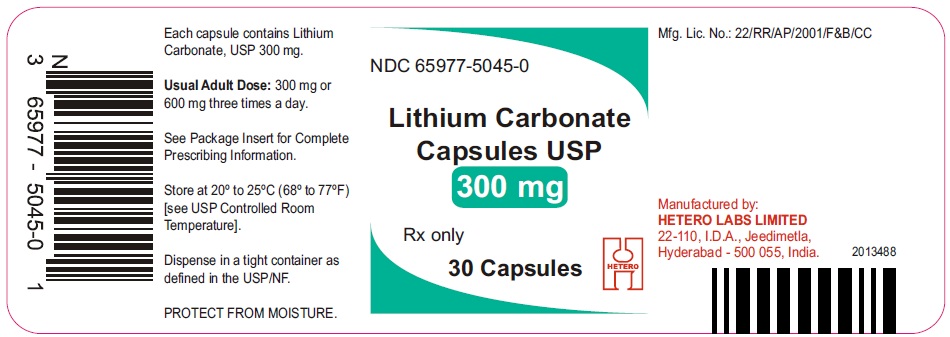
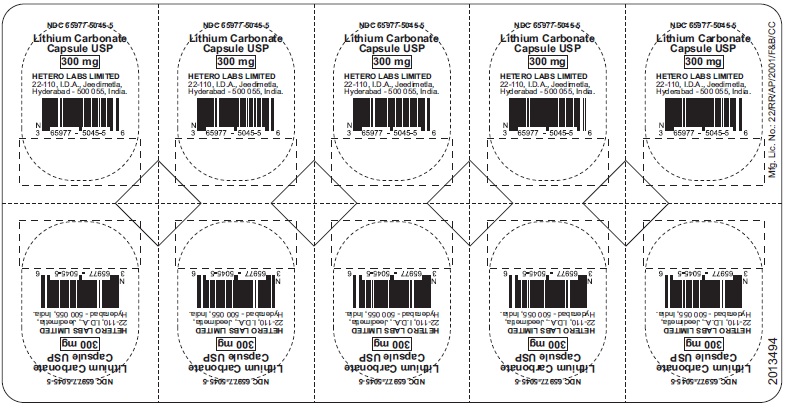
HOW SUPPLIED
Lithium Carbonate Capsules USP
150 mg White/White hard gelatin capsules (size 4)
Lithium Carbonate 150 mg Capsules are White/White size ‘4’ hard gelatin capsules, imprinted with ‘97’ on body and ‘H’ on cap, containing white to off-white powder. They are supplied in
Bottles of 30 Capsules (NDC: 65977-5044-0)
Bottles of 100 Capsules (NDC: 65977-5044-1)
Bottles of 500 Capsules (NDC: 65977-5044-2)
Bottles of 1000 Capsules (NDC: 65977-5044-3)
300 mg Pink/Pink hard gelatin capsules (size 1)
Lithium Carbonate 300 mg Capsules are Pink/Pink size ‘1’ hard gelatin capsules, imprinted with ‘98’ on body and ‘H’ on cap, containing white to off-white powder. They are supplied in
Bottles of 30 Capsules (NDC: 65977-5045-0)
Bottles of 100 Capsules (NDC: 65977-5045-1)
Bottles of 500 Capsules (NDC: 65977-5045-2)
Bottles of 1000 Capsules (NDC: 65977-5045-3)
Bottles of 5000 Capsules (NDC: 65977-5045-4)
Blister Pack of 3x10’s (NDC: 65977-5045-5)
600 mg Pink/White hard gelatin capsules (size ‘0EL’)
Lithium Carbonate 600 mg Capsules are Pink/White size ‘0EL’ hard gelatin capsules, imprinted with ‘141’ on body and ‘H’ on cap, containing white to off-white powder. They are supplied in
Bottles of 30 Capsules (NDC: 65977-5046-0)
Bottles of 100 Capsules (NDC: 65977-5046-1)
Bottles of 500 Capsules (NDC: 65977-5046-2)
Bottles of 1000 Capsules (NDC: 65977-5046-3)
Store and Dispense:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight container as defined in the USP/NF.

Revised: December 2011 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LITHIUM CARBONATE
lithium carbonate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65977-5044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 150 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C RED NO. 40 (UNII: WZB9127XOA) SHELLAC (UNII: 46N107B71O) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (white) Score no score Shape CAPSULE (capsule) Size 14mm Flavor Imprint Code 97;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65977-5044-0 30 in 1 BOTTLE 2 NDC: 65977-5044-1 100 in 1 BOTTLE 3 NDC: 65977-5044-2 500 in 1 BOTTLE 4 NDC: 65977-5044-3 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090702 09/25/2009 LITHIUM CARBONATE
lithium carbonate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65977-5045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 300 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C RED NO. 40 (UNII: WZB9127XOA) SHELLAC (UNII: 46N107B71O) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color PINK (pink) Score no score Shape CAPSULE (capsule) Size 19mm Flavor Imprint Code 98;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65977-5045-0 30 in 1 BOTTLE 2 NDC: 65977-5045-1 100 in 1 BOTTLE 3 NDC: 65977-5045-2 500 in 1 BOTTLE 4 NDC: 65977-5045-3 1000 in 1 BOTTLE 5 NDC: 65977-5045-4 5000 in 1 BOTTLE 6 NDC: 65977-5045-5 3 in 1 BOX, UNIT-DOSE 6 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090702 09/25/2009 LITHIUM CARBONATE
lithium carbonate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65977-5046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 600 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C RED NO. 40 (UNII: WZB9127XOA) SHELLAC (UNII: 46N107B71O) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color PINK (white) Score no score Shape CAPSULE (capsule) Size 23mm Flavor Imprint Code 141;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65977-5046-0 30 in 1 BOTTLE 2 NDC: 65977-5046-1 100 in 1 BOTTLE 3 NDC: 65977-5046-2 500 in 1 BOTTLE 4 NDC: 65977-5046-3 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090702 09/25/2009 Labeler - Hetero Drugs Limited (650229163) Registrant - Hetero Labs Limited (676162024) Establishment Name Address ID/FEI Business Operations Hetero Labs Limited 676162024 ANALYSIS(65977-5044, 65977-5046, 65977-5045) , MANUFACTURE(65977-5044, 65977-5045, 65977-5046)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.