KOSELUGO- selumetinib capsule
KOSELUGO by
Drug Labeling and Warnings
KOSELUGO by is a Prescription medication manufactured, distributed, or labeled by AstraZeneca Pharmaceuticals LP, AstraZeneca PLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KOSELUGO safely and effectively. See full prescribing information for KOSELUGO.
KOSELUGO (selumetinib) capsules, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
KOSELUGO is a kinase inhibitor indicated for the treatment of pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN). (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage is 25 mg/m2 taken orally twice daily on an empty stomach. Do not consume food 2 hours before each dose or 1 hour after each dose. (2.1)
- Reduce the recommended dosage to 20 mg/m2 orally twice daily for patients with moderate hepatic impairment (Child-Pugh B). The recommended dosage for use in patients with severe hepatic impairment (Child-Pugh C) has not been established. (2.2, 8.7)
DOSAGE FORMS AND STRENGTHS
Capsules: 10 mg and 25 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Cardiomyopathy: Assess ejection fraction prior to initiating treatment, every 3 months during the first year, then every 6 months thereafter and as clinically indicated. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.2, 5.1)
- Ocular Toxicity: Conduct ophthalmic assessments prior to initiating KOSELUGO, at regular intervals during treatment and for new or worsening visual changes. Permanently discontinue KOSELUGO for retinal vein occlusion (RVO). Withhold KOSELUGO for retinal pigment epithelial detachment (RPED), monitor with optical coherence tomography assessments until resolution, and resume at reduced dose. (2.2, 5.2)
- Gastrointestinal Toxicity: Advise patients to start an anti-diarrheal agent immediately after the first episode of loose stool and to increase fluid intake. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.2, 5.3)
- Skin Toxicity: Monitor for severe skin rashes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.2, 5.4)
- Increased Creatinine Phosphokinase (CPK): Increased CPK and rhabdomyolysis can occur. Obtain serum CPK prior to initiating KOSELUGO, periodically during treatment, and as clinically indicated. If increased CPK occurs, evaluate for rhabdomyolysis or other causes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.2, 5.5)
- Increased Vitamin E Levels and Risk of Bleeding: KOSELUGO capsules contain vitamin E and daily intake of vitamin E that exceeds the recommended or safe limits may increase the risk of bleeding. An increased risk of bleeding may occur in patients coadministered vitamin-K antagonists or anti-platelet agents. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (≥ 40%) are: vomiting, rash (all), abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, acneiform rash, stomatitis, headache, paronychia, and pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong or Moderate CYP3A4 Inhibitors or Fluconazole: Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the dose of KOSELUGO. (2.4, 7.1)
- Strong or Moderate CYP3A4 Inducers: Avoid concomitant use of strong and moderate CYP3A4 inducers. (7.1)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
2.3 Dosage Modifications for Hepatic Impairment
2.4 Dosage Modifications for Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
5.2 Ocular Toxicity
5.3 Gastrointestinal Toxicity
5.4 Skin Toxicity
5.5 Increased Creatinine Phosphokinase
5.6 Increased Levels of Vitamin E and Risk of Bleeding
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on KOSELUGO
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of KOSELUGO is 25 mg/m2 orally twice daily (approximately every 12 hours) until disease progression or unacceptable toxicity.
Take KOSELUGO on an empty stomach. Do not consume food 2 hours before each dose or 1 hour after each dose [see Clinical Pharmacology (12.3)]. The recommended dose of KOSELUGO based on body surface area (BSA) is shown in Table 1.
Table 1 Recommended Dosage Based on Body Surface Area - * The recommended dosage for patients with a BSA less than 0.55m2 has not been established.
Body Surface Area*
Recommended Dosage
0.55 – 0.69 m2
20 mg in the morning and 10 mg in the evening
0.70 – 0.89 m2
20 mg twice daily
0.90 – 1.09 m2
25 mg twice daily
1.10 – 1.29 m2
30 mg twice daily
1.30 – 1.49 m2
35 mg twice daily
1.50 – 1.69 m2
40 mg twice daily
1.70 – 1.89 m2
45 mg twice daily
≥1.90 m2
50 mg twice daily
Swallow KOSELUGO capsules whole with water. Do not chew, dissolve or open capsule.
Do not administer to patients who are unable to swallow a whole capsule.
Do not take a missed dose of KOSELUGO unless it is more than 6 hours until the next scheduled dose.
If vomiting occurs after KOSELUGO administration, do not take an additional dose, but continue with the next scheduled dose.
2.2 Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions are provided in Table 2.
Table 2 Recommended Dose Reductions for KOSELUGO for Adverse Reactions - * Permanently discontinue KOSELUGO in patients unable to tolerate KOSELUGO after two dose reductions.
Body Surface Area
First Dose Reduction
(mg/dose)
Second Dose Reduction*
(mg/dose)
Morning
Evening
Morning
Evening
0.55 – 0.69 m2
10
10
10 once daily
0.70 – 0.89 m2
20
10
10
10
0.90 – 1.09 m2
25
10
10
10
1.10 – 1.29 m2
25
20
20
10
1.30 – 1.49 m2
25
25
25
10
1.50 – 1.69 m2
30
30
25
20
1.70 – 1.89 m2
35
30
25
20
≥1.90 m2
35
35
25
25
Dose modifications for adverse reactions are presented Table 3.
Table 3 Recommended Dosage Modifications for KOSELUGO for Adverse Reactions Severity of Adverse Reaction Recommended Dosage Modifications for KOSELUGO Cardiomyopathy [see Warnings and Precautions (5.1)]
- Asymptomatic decrease in left ventricular ejection (LVEF) of 10% or greater from baseline and less than lower level of normal
Withhold until resolution. Resume at reduced dose.
- Symptomatic decreased LVEF
- Grade 3 or 4 decreased LVEF
Permanently discontinue.
Ocular Toxicity [see Warnings and Precautions (5.2)]
- Retinal Pigment Epithelial Detachment (RPED)
Withhold until resolution. Resume at reduced dose.
- Retinal vein occlusion (RVO)
Permanently discontinue.
Gastrointestinal Toxicity[see Warnings and Precautions (5.3)]
- Grade 3 Diarrhea
Withhold until improved to Grade 0 or 1. Resume at same dose. Permanently discontinue if no improvement within 3 days.
- Grade 4 Diarrhea
Permanently discontinue.
- Grade 3 or 4 Colitis
Permanently discontinue.
Skin Toxicity [see Warnings and Precautions (5.4)]
- Grade 3 or 4
Withhold until improvement. Resume at reduced dose.
Increased Creatinine Phosphokinase (CPK) [see Warnings and Precautions (5.5)]
- Grade 4 Increased CPK
- Any Increased CPK and myalgia
Withhold until improved to Grade 0 or 1. Resume at reduced dose. Permanently discontinue if no improvement within 3 weeks.
- Rhabdomyolysis
Permanently discontinue.
Other Adverse Reactions [see Adverse Reactions (6.1)]
- Intolerable Grade 2
- Grade 3
Withhold KOSELUGO until improve to Grade 0 or 1. Resume at reduced dose.
- Grade 4
Withhold KOSELUGO until improved to Grade 0 or 1. Resume at reduced dose. Consider discontinuation.
* Per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03
2.3 Dosage Modifications for Hepatic Impairment
Reduce the recommended dosage of KOSELUGO to 20 mg/m2 orally twice daily in patients with moderate hepatic impairment (Child-Pugh B). The recommended dosage of KOSELUGO for use in patients with severe hepatic impairment (Child-Pugh C) has not been established [see Use in Specific Populations (8.7)].
Table 4 Recommended Dosage of KOSELUGO for Moderate Hepatic Impairment Body Surface Area Moderate Hepatic Impairment
(Child-Pugh B)
(mg/dose)Morning Evening 0.55 – 0.69 m2
10
10
0.70 – 0.89 m2
20
10
0.90 – 1.09 m2
20
20
1.10 – 1.29 m2
25
25
1.30 – 1.49 m2
30
25
1.50 – 1.69 m2
35
30
1.70 – 1.89 m2
35
35
≥ 1.90 m2
40
40
2.4 Dosage Modifications for Drug Interactions
Strong or Moderate CYP3A4 Inhibitors or Fluconazole
Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the KOSELUGO dosage as recommended in Table 5. After discontinuation of the strong or moderate CYP3A4 inhibitor or fluconazole for 3 elimination half-lives, resume the KOSELUGO dose that was taken prior to initiating the inhibitor or fluconazole [see Drug Interactions (7.1)].
Table 5 Recommended Dosage of KOSELUGO for Coadministration with Strong or Moderate CYP3A4 Inhibitors or Fluconazole Body Surface Area If the current dosage is 25 mg/m2 twice daily, reduce to 20 mg/m2 twice daily
(mg/dose)If the current dosage is 20 mg/m2 twice daily, reduce to 15 mg/m2 twice daily
(mg/dose)Morning Evening Morning Evening 0.55 – 0.69 m2
10
10
10 mg once a day
0.70 – 0.89 m2
20
10
10
10
0.90 – 1.09 m2
20
20
20
10
1.10 – 1.29 m2
25
25
25
10
1.30 – 1.49 m2
30
25
25
20
1.50 – 1.69 m2
35
30
25
25
1.70 – 1.89 m2
35
35
30
25
≥ 1.90 m2
40
40
30
30
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiomyopathy
Cardiomyopathy, defined as a decrease in left ventricular ejection fraction (LVEF) ≥ 10% below baseline, occurred in 23% of 74 pediatric patients who received KOSELUGO in SPRINT [see Adverse Reactions (6.1)]. Four percent of patients experienced decreased LVEF below the institutional lower limit of normal (LLN). Grade 3 decreased LVEF occurred in one patient and resulted in dose reduction. All patients with decreased LVEF were asymptomatic and identified during routine echocardiography. Decreased LVEF resolved in 71% of these patients.
Left ventricular dysfunction or decreased LVEF resulting in permanent discontinuation of KOSELUGO occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO. Decreased LVEF resulting in permanent discontinuation of KOSELUGO occurred in a pediatric population with NF1 in an expanded access program.
The safety of KOSELUGO has not been established in patients with a history of impaired LVEF or a baseline ejection fraction that is below the institutional LLN
Assess ejection fraction by echocardiogram prior to initiating treatment, every 3 months during the first year of treatment, every 6 months thereafter, and as clinically indicated. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.2)]. In patients who interrupt KOSELUGO for decreased LVEF, obtain an echocardiogram or a cardiac MRI every 3 to 6 weeks. Upon resolution of decreased LVEF to greater than or equal to the institutional LLN, obtain an echocardiogram or a cardiac MRI every 2 to 3 months or as directed by the cardiologist.
5.2 Ocular Toxicity
Blurred vision, photophobia, cataracts, and ocular hypertension occurred in 15% of 74 pediatric patients receiving KOSELUGO in SPRINT. Blurred vision resulted in dose interruption in 2.7% of patients. Ocular toxicity resolved in 82% of 11 patients.
Serious ocular toxicities including retinal vein occlusion (RVO) and retinal pigment epithelial detachment (RPED), occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents. RPED occurred in the pediatric population during treatment with single agent KOSELUGO and resulted in permanent discontinuation.
Conduct comprehensive ophthalmic assessments prior to initiating KOSELUGO, at regular intervals during treatment, and for new or worsening visual changes. Permanently discontinue KOSELUGO in patients with RVO. Withhold KOSELUGO in patients with RPED, follow up with optical coherence tomography assessments every 3 weeks until resolution, and resume KOSELUGO at a reduced dose. For other ocular toxicities, withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of the adverse reaction [see Dosage and Administration (2.2)].
5.3 Gastrointestinal Toxicity
Diarrhea occurred in 77% of 74 pediatric patients who received KOSELUGO in SPRINT, including Grade 3 in 15% of patients. Diarrhea resulting in permanent discontinuation occurred in 1.4% of patients. Diarrhea resulting in dose interruption or dose reduction occurred in 15% and 1.4% of patients, respectively. The median time to first onset of diarrhea was 17 days and the median duration was 2 days.
Serious gastrointestinal toxicities, including perforation, colitis, ileus, and intestinal obstruction, occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents. Colitis occurred in an unapproved population of pediatric patients with multiple tumor types who received KOSELUGO as a single agent.
Advise patients to start an anti-diarrheal agent (e.g., loperamide) immediately after the first episode of unformed, loose stool and to increase fluid intake during diarrhea episodes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.2)].
5.4 Skin Toxicity
Rash occurred in 91% of 74 pediatric patients who received KOSELUGO in SPRINT. The most frequent rashes included dermatitis acneiform (54%), maculopapular rash (39%), and eczema (28%). Grade 3 rash occurred in 8% of patients. Rash resulted in dose interruption in 11% of patients and dose reduction in 4% of patients.
Other skin toxicities, including severe palmar-plantar erythrodysesthesia syndrome (PPES), occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents.
Monitor for severe skin rashes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3)].
5.5 Increased Creatinine Phosphokinase
Increased creatinine phosphokinase (CPK) occurred in 76% of 74 pediatric patients who received KOSELUGO in SPRINT, including Grade 3 or 4 in 9% of patients. Increased CPK resulted in dose reduction in 7% of patients. Increased CPK concurrent with myalgia occurred in 8% of patients, including one patient who permanently discontinued KOSELUGO for myalgia.
Rhabdomyolysis occurred in an unapproved adult population who received KOSELUGO as a single agent.
Obtain serum CPK prior to initiating KOSELUGO, periodically during treatment, and as clinically indicated. If increased CPK occurs, evaluate patients for rhabdomyolysis or other causes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3)].
5.6 Increased Levels of Vitamin E and Risk of Bleeding
KOSELUGO capsules contain vitamin E (10 mg capsules contain 32 mg vitamin E as the excipient, D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS); while KOSELUGO 25 mg capsules contain 36 mg vitamin E as TPGS). Vitamin E can inhibit platelet aggregation and antagonize vitamin K-dependent clotting factors. Daily vitamin E intake that exceeds the recommended or safe limits may increase the risk of bleeding. Supplemental vitamin E is not recommended if daily vitamin E intake (including the amount of vitamin E in KOSELUGO and supplement) will exceed the recommended or safe limits.
An increased risk of bleeding in patients may occur in patients who are coadministered vitamin-K antagonists or anti-platelet antagonists with KOSELUGO. Monitor for bleeding in these patients. Increase international normalized ratio (INR) monitoring, as appropriate, in patients taking a vitamin-K antagonist. Perform anticoagulant assessments, including INR or prothrombin time, more frequently and adjust the dose of vitamin K antagonists or anti-platelet agents as appropriate [see Drug Interactions (7.1)].
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, KOSELUGO can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of selumetinib to mice during organogenesis caused reduced fetal weight, adverse structural defects, and effects on embryo-fetal survival at approximate exposures > 5 times the human exposure at the clinical dose of 25 mg/m2 twice daily. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cardiomyopathy [see Warnings and Precautions (5.1)]
- Ocular toxicity [see Warnings and Precautions (5.2)]
- Gastrointestinal toxicity [see Warnings and Precautions (5.3)]
- Skin toxicity [see Warnings and Precautions (5.4)]
- Increased creatinine phosphokinase [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS reflects exposure to KOSELUGO in 74 pediatric patients who received a dosage ranging from 20 mg/m2 to 30 mg/m2 orally twice daily in SPRINT. Among these patients, the duration of KOSELUGO exposure, including dose interruptions, was 12 months or longer (91%), more than 2 years (74%), or more than 4 years (23%). The WARNINGS AND PRECAUTIONS also includes additional data from adult and pediatric patients who received KOSELUGO administered at various doses across a range of tumors in other clinical trials.
Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN)
The safety of KOSELUGO was evaluated in SPRINT Phase II Stratum 1 [see Clinical Studies (14)]. Eligible patients were 2-18 years of age with NF1 who had inoperable PN that was causing significant morbidity. Patients were excluded for abnormal LVEF, uncontrolled hypertension (blood pressure > the 95th percentile for age, height, and sex), any current or past history of RVO or RPED, intraocular pressure > 21 mmHg (or upper limit of normal adjusted by age), uncontrolled glaucoma, and inability to swallow whole capsules. Patients received KOSELUGO 25 mg/m2 orally twice daily (n=50). Among these patients, 88% were exposed for 12 months or longer and 66% were exposed for greater than 2 years.
Serious adverse reactions occurred in 24% of patients who received KOSELUGO. Serious adverse reactions that occurred in 2 or more patients were anemia, hypoxia and diarrhea.
Permanent discontinuation due to an adverse reaction occurred in 12% of patients who received KOSELUGO. Adverse reactions resulting in permanent discontinuation of KOSELUGO included increased creatinine, increased weight, diarrhea, paronychia, malignant peripheral nerve sheath tumor, acute kidney injury, and skin ulcer.
Dosage interruptions and dose reductions due to adverse reactions occurred in 80% and 24% of patients who received KOSELUGO, respectively. Adverse reactions requiring a dosage interruption or reduction in ≥ 5% of patients were vomiting, paronychia, diarrhea, nausea, abdominal pain, rash, skin infection, influenza-like illness, pyrexia and weight gain.
The most common adverse reactions (≥ 40%) were vomiting, rash (all), abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, acneiform rash, stomatitis, headache, paronychia, and pruritis.
Table 6 presents the adverse reactions in SPRINT Phase II Stratum 1.
Table 6 Adverse Reactions (≥ 20%) in Patients Who Received KOSELUGO in SPRINT Phase II Stratum 1
Adverse Reaction KOSELUGO
N=50All Grades
(%)Grade ≥ 3
(%)*- * Abdominal pain includes abdominal pain; abdominal pain upper
- † Stomatitis includes stomatitis; mouth ulceration
- ‡ Rash (all) includes dermatitis acneiform; rash maculo-papular; erythema; rash pustular; rash; urticaria; exfoliative rash; rash pruritic; rash erythematous
- § Rash (acneiform) includes dermatitis acneiform
- ¶ Paronychia includes paronychia, nail infection
- # Dermatitis includes dermatitis; dermatitis atopic; dermatitis diaper; eczema; seborrheic dermatitis; skin irritation
- Þ Hair changes include alopecia, hair color change
- ß Musculoskeletal pain includes pain in extremity; back pain; neck pain; musculoskeletal pain
- à Fatigue includes fatigue, malaise
- è Edema includes peripheral swelling, edema, localized edema
- ð Skin infection includes skin infection; abscess; cellulitis; impetigo; staphylococcal skin infection
Gastrointestinal
Vomiting
82
6
Abdominal pain*
76
0
Diarrhea
70
16
Nausea
66
2
Stomatitis†
50
0
Constipation
34
0
Skin and Subcutaneous Tissue
Rash (all)‡
80
6
Dry skin
60
0
Rash acneiform§
50
4
Paronychia¶
48
6
Pruritus
46
0
Dermatitis#
36
4
Hair changesÞ
32
0
Musculoskeletal and Connective Tissue
Musculoskeletal painß
58
0
General
Fatigueà
56
0
Pyrexia
56
8
Edemaè
20
0
Nervous System
Headache
48
2
Respiratory, Thoracic and Mediastinal
Epistaxis
28
0
Renal and Urinary System
Hematuria
22
2
Proteinuria
22
0
Metabolism and Nutrition
Decreased appetite
22
0
Cardiac System
Decreased ejection fraction
22
0
Sinus tachycardia
20
0
Infections
Skin infectionð
20
2
* All events were Grade 3.
Clinically relevant adverse reactions that occurred < 20% of patients include:
- Eye: visual impairment
- Gastrointestinal Disorders: dry mouth
- General Disorders: facial edema, including periorbital edema and face edema
- Metabolism and Nutrition: increased weight
- Renal and Urinary System: acute kidney injury
- Respiratory, Thoracic & Mediastinal: dyspnea, including exertional dyspnea and dyspnea at rest
- Vascular: hypertension
Table 7 presents the laboratory abnormalities in SPRINT Phase II Stratum 1.
Table 7 Select Laboratory Abnormalities (≥ 15%) Worsening from Baseline in Patients Who Received KOSELUGO in SPRINT Phase II Stratum 1 Laboratory Abnormality KOSELUGO All Grades (%)* Grade ≥ 3 (%) - * The denominator used to calculate the rate varied from 39 to 49 based on the number of patients with a baseline value and at least one post-treatment value.
- † Includes one Grade 4 increased CPK and one Grade 4 increased potassium.
Chemistry
- Increased creatinine phosphokinase (CPK)
79
7†
- Decreased albumin
51
0
- Increased aspartate aminotransferase (AST)
41
2
- Increased aspartate aminotransferase (AST)
35
4
- Increased lipase
32
5
- Increased potassium
27
4
- Decreased potassium
18
2§
- Increased alkaline phosphatase
18
0
- Increased amylase
18
0
- Increased sodium
18
0
- Decreased sodium
16
0
Hematology
- Decreased hemoglobin
41
4
- Decreased neutrophils
33
4
- Decreased lymphocytes
20
2
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on KOSELUGO
Strong or Moderate CYP3A4 Inhibitors or Fluconazole
Clinical Impact
- Concomitant use of KOSELUGO with a strong or moderate CYP3A4 inhibitor or fluconazole increased selumetinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions.
Management
- Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce KOSEULGO dosage [see Dosage and Administration (2.4)].
Strong or Moderate CYP3A4 Inducers
Clinical Impact
- Concomitant use of KOSELUGO with a strong or moderate CYP3A4 inducer decreased selumetinib plasma concentrations [see Clinical Pharmacology (12.3)], which may reduce KOSELUGO efficacy.
Management
- Avoid concomitant use of strong or moderate CYP3A4 inducers with KOSELUGO.
Vitamin E
Clinical Impact
- KOSELUGO contains vitamin E and daily vitamin E intake that exceeds the recommended or safe limits may increase the risk of bleeding. An increased risk of bleeding may occur in patients taking a vitamin-K antagonist or an anti-platelet agent with KOSELUGO.
Management
- Supplemental vitamin E is not recommended if daily vitamin E intake (including the amount of vitamin E in KOSELUGO and supplement) will exceed the recommended or safe limits.
- Monitor for bleeding in patients coadministered a vitamin-K antagonist or an antiplatelet agent with KOSELUGO. Increase INR monitoring, as appropriate, in patients taking a vitamin-K antagonist [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], KOSELUGO can cause fetal harm when administered to a pregnant woman. There are no available data on the use of KOSELUGO in pregnant women to evaluate drug-associated risk. In animal reproduction studies, administration of selumetinib to mice during organogenesis caused reduced fetal weight, adverse structural defects, and effects on embryofetal survival at exposures approximately > 5 times the human exposure at the clinical dose of 25 mg/m2 twice daily (see Data). Advise pregnant women of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryo-fetal development studies in mice at doses > 2.5 mg/kg twice daily (~5 times the human exposure based on area under the curve [AUC] at the clinical dose of 25 mg/m2 twice daily), selumetinib caused increases in post-implantation loss, a reduction in mean fetal and litter weights, and an increased occurrence of open eye and cleft palate, but did not induce significant maternal toxicity.
Administration of selumetinib to pregnant mice from gestation Day 6 through lactation Day 20 resulted in reduced pup body weights and fewer pups met the pupil constriction criterion on day 21 post-partum. The incidence of malformations (e.g. prematurely open eye(s) and cleft palate) was increased even at the lowest dose of 0.5 mg/kg twice daily (maternal maximal concentration [Cmax] of ~0.6 times the human Cmax at the clinical dose of 25 mg/m2 twice daily).
8.2 Lactation
Risk Summary
There are no data on the presence of selumetinib or its active metabolite in human milk or their effects on the breastfed child or milk production. Selumetinib and its active metabolite were present in the milk of lactating mice (see Data). Due to the potential for adverse reactions in a breastfed child, advise women not to breastfeed during treatment with KOSELUGO and for 1 week after the last dose.
Data
Animal Data
Selumetinib and its active metabolite were present in milk from mice dosed with selumetinib throughout gestation and lactation, with a mean plasma/milk ratio of 1.5 in lactating dams dosed at 5 mg/kg twice daily. Administration of selumetinib to dams during gestation and early lactation was associated with adverse events in pups, including reduced growth rates and incidence of malformations [see Use in Specific Populations (8.1)].
8.3 Females and Males of Reproductive Potential
KOSELUGO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating KOSELUGO [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose.
8.4 Pediatric Use
The safety and effectiveness have been established in pediatric patients 2 years of age and older with NF1 who have inoperable PN and the information on this use is discussed throughout the labeling. The safety and effectiveness of KOSELUGO have not been established in pediatric patients younger than 2 years of age.
Animal Toxicity Data
In 3-month general toxicology studies, male rats receiving selumetinib at doses ≥ 10 mg/kg daily (~60 times the human exposure based on AUC at the clinical dose of 25 mg/m2 twice daily) showed growth plate dysplasia.
8.6 Renal Impairment
No dose adjustment is recommended in patients with renal impairment or those with End Stage Renal Disease [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Selumetinib exposures increased in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)]. Reduce the dose of KOSELUGO for patients with moderate hepatic impairment (Child-Pugh B). A recommended dosage of KOSELUGO for use in patients with severe hepatic impairment (Child-Pugh C) has not been established [see Dosage and Administration (2.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
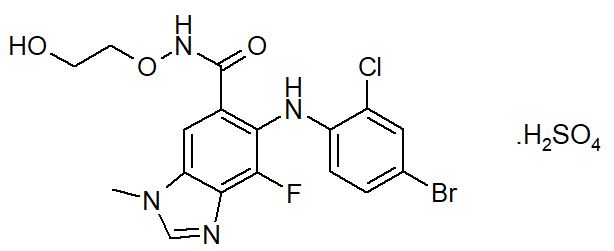
Selumetinib is a kinase inhibitor. The chemical name is 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-6-[(2-hydroxyethoxy)carbamoyl]-1-methyl-1H-benzimidazol-3-ium hydrogen sulfate. The molecular formula for selumetinib sulfate is C17H17BrClFN4O7S and the relative molecular mass is 555.76 g/mol. Selumetinib sulfate has the following structural formula:
Selumetinib sulfate is a white to yellow monomorphic crystalline powder that exhibits a pH dependent solubility. Selumetinib sulfate is freely soluble at pH < 1.5, sparingly soluble in the pH range at 1.5 to 3 and slightly soluble at pH > 3. Selumetinib sulfate has two ionizable functions with pKa values of 2.8 and 8.4.
KOSELUGO (selumetinib) 10 mg capsules for oral use, contain 10 mg selumetinib (equivalent to 12.1 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains hypromellose, carrageenan, potassium chloride, titanium dioxide, carnauba wax, and purified water. The capsule is imprinted with black ink that contains shellac, iron oxide black, propylene glycol and ammonium hydroxide.
KOSELUGO (selumetinib) 25 mg capsules for oral use, contain 25 mg selumetinib (equivalent to 30.25 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains hypromellose, carrageenan, potassium chloride, titanium dioxide, FD&C blue 2, ferric oxide yellow, purified water, carnauba wax, and/or corn starch. The capsule is imprinted with black ink that contains ferric oxide red, ferric oxide yellow, FD&C Blue 2 aluminum lake, carnauba wax, shellac, and glyceryl monooleate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Selumetinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. Both MEK and ERK are critical components of the RAS-regulated RAF-MEK-ERK pathway, which is often activated in different types of cancers.
In genetically modified mouse models of NF1 that generate neurofibromas that recapitulate the genotype and phenotype of human NF1, oral dosing of selumetinib inhibited ERK phosphorylation, and reduced neurofibroma numbers, volume, and proliferation.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of KOSELUGO have not been fully characterized.
Cardiac Electrophysiology
At a dose 1.5 times the maximum recommended dose, KOSELUGO does not prolong the QT/QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
At the recommended dosage of 25 mg/m2 twice daily in pediatric patients (2 to ≤ 18 years old), the mean maximum plasma concentration (Cmax) (coefficient of variation [CV%]) following the first dose and at steady state was 731 (62%) ng/mL and 798 (52%) ng/mL, respectively. The mean area under the plasma drug concentration curve (AUC0-12h) following the first dose was 2009 (35%) ngh/mL and the AUC0-6h at steady state was 1958 (41%) ngh/mL. Selumetinib AUC and Cmax increases proportionally over a dose range from 20 mg/m2 to 30 mg/m2 (0.8 to 1.2 times the recommended dose). The accumulation was 1.1-fold following administration of KOSELUGO 25 mg/m2 twice daily.
Absorption
The mean absolute oral bioavailability of selumetinib was 62% in healthy adults. The median time to peak plasma concentrations (Tmax) at steady-state in pediatric patients was 1 to 1.5 hours.
Effect of Food
Mean Cmax and AUC of selumetinib decreased by 50% and 16%, respectively, following a high-fat meal (1000 calories, 50% fat) in healthy adults administered a single-dose of 75 mg (1.5 times the approved maximum recommended dosage). Tmax was delayed by approximately 1.5 hours following a high-fat meal.
Selumetinib Cmax and AUC decreased by 60% and 38%, respectively, following a low-fat meal (400 calories, 25% fat) in healthy adults administered a single-dose of 50 mg. Tmax was delayed by approximately 0.9 hours following a low-fat meal.
Distribution
The mean apparent volume of distribution at steady state (Vss) of selumetinib across a dose range of 20 mg/m2 to 30 mg/m2 (0.8 to 1.2 times the recommended dosage) ranged from 78 L to 171 L in pediatric patients.
The plasma protein binding was 98.4% in humans in vitro. Selumetinib binds to serum albumin (96%) and α-1 acid glycoprotein (< 35%).
Elimination
In pediatric patients, selumetinib had an apparent oral clearance (CL/F) of 8.8 L/hr and a mean elimination half-life of approximately 6.2 hours following a dose of 25 mg/m2.
Metabolism
Selumetinib is primarily metabolized by CYP3A4 and to a lesser extent by CYP2C19, CYP1A2, CYP2C9, CYP2E1, and CYP3A5. Selumetinib also undergoes glucuronidation by UGT1A1 and UGT1A3. It is estimated that 56% of the observed intrinsic clearance of selumetinib could be attributed to CYP metabolism and about 29% attributed to direct glucuronidation by UGT enzymes in vitro. The active metabolite, N-desmethyl selumetinib, is generated by CYP2C19 and CYP1A2 with additional contribution by CYP2C9 and CYP2A6, and metabolized through the same routes as selumetinib.
N-desmethyl selumetinib represents less than 10% of selumetinib levels in human plasma, but is approximately 3 to 5 times more potent than the parent compound, contributing to about 21% to 35% of the overall pharmacologic activity.
Excretion
After a single oral dose of radiolabeled selumetinib 75 mg (1.5 times the recommended dose) to healthy adults, 59% of the dose was recovered in feces (19% as unchanged) and 33% in urine (< 1% as parent).
Specific Populations
Racial or Ethnic Groups
No clinically meaningful effect on the pharmacokinetics of selumetinib or N-desmethyl selumetinib were observed based on race (White, Asian, Black).
Patients with Renal Impairment
Following administration of a single dose of 50 mg, selumetinib exposures were similar in subjects with end stage renal disease (CLcr < 15 mL/min) who required dialysis compared to subjects with normal renal function (CLcr ≥ 90 mL/min).
Patients with Hepatic Impairment
Following administration of a single-dose of selumetinib, dose normalized total AUC0-INF decreased by 14% in subjects with mild hepatic impairment (Child-Pugh A), and increased by 59% in subjects with moderate hepatic impairment (Child-Pugh B) and by 57% in subjects with severe hepatic impairment (Child-Pugh class C) compared to subjects with normal hepatic function. Selumetinib unbound AUC0-INF decreased by 31% in subjects with mild hepatic impairment (Child-Pugh A), and increased by 41% in subjects with moderate hepatic impairment (Child-Pugh B), and 3.2-fold in subjects with severe hepatic impairment (Child-Pugh C) compared to subjects with normal hepatic function.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Strong or Moderate CYP3A4 Inhibitors: Concomitant use of itraconazole (strong CYP3A4 inhibitor) increased selumetinib AUC by 49% and Cmax by 19%. Concomitant use of erythromycin (moderate CYP3A4 inhibitor) is predicted to increase selumetinib AUC by 41% and Cmax by 23%.
Effect of Fluconazole: Concomitant use of fluconazole (strong CYP2C19 inhibitor and moderate CYP3A4 inhibitor) increased selumetinib AUC by 53% and Cmax by 26%.
Effect of Strong or Moderate CYP3A4 Inducers: Concomitant use of rifampicin (strong CYP3A4 inducer) decreased selumetinib AUC by 51% and Cmax by 26%. Concomitant use of efavirenz (moderate CYP3A4 inducer) is predicted to decrease selumetinib AUC by 38% and Cmax by 22%.
In Vitro Studies
CYP Enzymes: Selumetinib does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, or CYP2E1. Selumetinib does not induce CYP3A4, CYP1A2, or CYP2B6.
Transporter Systems: Selumetinib does not inhibit breast cancer resistance protein (BCRP), P-glycoprotein (P-gp), OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2K transporters.
Selumetinib is a substrate of BCRP and P-gp transporters.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Selumetinib was not carcinogenic in a 6-month study in rasH2 transgenic mice at exposures 24 times (males) and 36 times (females) and in 2-year carcinogenicity study in rats at exposures 20 times (male) and 15 times the human exposure (AUC) at the clinical dose of 25 mg/m2.
Mutagenicity
Selumetinib was not mutagenic or clastogenic in vitro. Selumetinib did result in an increase in micronucleated immature erythrocytes (chromosome aberrations) in mouse micronucleus studies, predominantly via an aneugenic mode of action, but at doses > 160 mg/kg (~38 times the human Cmax at the clinical dose of 25 mg/m2).
Impairment of Fertility
In a 6-month mouse study, selumetinib did not affect male mating performance at any dose up to 20 mg/kg twice daily (approximately 33 times the human exposure based on AUC at the clinical dose of 25 mg/m2 twice daily). In female mice exposed to selumetinib at 12.5 mg/kg twice daily, mating performance and fertility were not affected. The NOAEL for both maternal toxicity and effects on reproductive performance was 2.5 mg/kg twice daily (approximately 5 times the human exposure based on AUC at the clinical dose of 25 mg/m2 twice daily).
13.2 Animal Toxicology and/or Pharmacology
In a 26-week repeat-dose toxicology study, selumetinib at a dose of 20 mg/kg (approximately 33 times the human exposure based on AUC at the clinical dose of 25 mg/m2 twice daily) led to significant urinary tract obstruction as well as inflammation and luminal hemorrhage of the urethra leading to early death in male mice.
-
14 CLINICAL STUDIES
14.1 Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN)
The efficacy of KOSELUGO was evaluated in SPRINT Phase II Stratum 1, an open-label, multicenter, single arm trial (NCT01362803). Eligible patients were required to have NF1 with inoperable PN, defined as a PN that could not be completely removed without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness, or high vascularity of the PN. Patients were also required to have significant morbidity related to the target PN. Morbidities that were present in ≥ 20% of patients included disfigurement, motor dysfunction, pain, airway dysfunction, visual impairment, and bladder/bowel dysfunction. Patients received KOSELUGO 25 mg/m2 orally twice daily until disease progression or unacceptable toxicity.
The major efficacy outcome measure was overall response rate (ORR), defined as the percentage of patients with complete response (defined as disappearance of the target PN) or confirmed partial response (defined as ≥ 20% reduction in PN volume confirmed at a subsequent tumor assessment within 3-6 months). The target PN, defined as the PN that caused relevant clinical symptoms or complications (PN-related morbidities), was evaluated for response rate using centrally read volumetric magnetic resonance imaging (MRI) analysis per Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria. Tumor response was evaluated at baseline and while on treatment after every 4 cycles for 2 years, and then every 6 cycles. An additional efficacy outcome measure was duration of response (DoR).
A total of 50 pediatric patients received KOSELUGO. The median age was 10.2 years (range 3.5 to 17.4 years); 60% were male; and 84% were White, 8% were Black and 2% were Asian.
Efficacy results are provided in Table 8. The median time to onset of response was 7.2 months (range: 3.3 months to 1.6 years).
Table 8 Efficacy Results from SPRINT Phase II Stratum 1* Efficacy Parameter SPRINT
N = 50- * The ORR assessment was conducted by a single NCI reviewer who was a SPRINT investigator and who evaluated all PN imaging from patients enrolled at all trial sites.
- † Responses required confirmation at least 3 months after the criteria for first response were met.
- ‡ Complete response: disappearance of the target lesion; Partial response: decrease in target PN volume by ≥ 20% compared to baseline.
Overall Response Rate †
- Overall Response Rate, n (%)
33 (66%)
- 95% CI
(51, 79)
- Complete Response‡
0
- Confirmed Partial Response, n (%)‡
33 (66%)
Duration of Response
- DoR ≥ 12 months, n (%)
27 (82%)
CI – confidence interval, DoR – duration of response.
An independent centralized review of tumor response per REiNS criteria resulted in an ORR of 44% (95% CI: 30, 59).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Strength
Description
Capsules per Bottle
NDC Number
10 mg
White, opaque, hard capsule, banded and marked with “SEL 10” in black ink.
60
0310-0610-60
25 mg
Blue, opaque, hard capsule, banded and marked with “SEL 25” in black ink.
60
0310-0625-60
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Dispense in original bottle. Do not remove desiccant. Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Cardiomyopathy
Advise patients and caregivers that KOSELUGO can cause a reduction in LVEF and to immediately report any signs or symptoms of cardiomyopathy to their healthcare provider [see Warnings and Precautions (5.1)].
Ocular Toxicity
Advise patients and caregivers that KOSELUGO can cause ocular toxicity that can lead to blindness and to contact their healthcare provider if the patient experiences any changes in their vision [see Warnings and Precautions (5.2)].
Gastrointestinal Toxicity
Advise patients and caregivers that KOSELUGO can cause diarrhea and to contact their healthcare provider at the onset of diarrhea [see Warnings and Precautions (5.3)].
Skin Toxicity
Advise patients and caregivers that KOSELUGO can cause serious skin toxicities and to contact their healthcare provider for severe skin changes [see Warnings and Precautions (5.4)].
Increased Creatinine Phosphokinase
Advise patients and caregivers that KOSELUGO can cause increased CPK and to report any signs and symptoms of muscle pain or weakness to their healthcare provider [see Warnings and Precautions (5.5)].
Increased Vitamin E Levels and Risk of Bleeding
Advise patients and caregivers to notify their healthcare provider if they are taking a supplement containing vitamin E, a vitamin-K antagonist or an anti-platelet agent [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Advise males with female partners of reproductive potential to use effective contraception during treatment with KOSELUGO and for at least 1 week after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with KOSELUGO and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Drug Interactions
Advise patients and caregivers to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Inform patients to avoid St. John’s wort, grapefruit or grapefruit juice while taking KOSELUGO [see Drug Interactions (7)].
Dosing and Administration
Inform patients and caregivers on how to take KOSELUGO with food and what to do for missed or vomited doses [see Dosage and Administration (2.1)].
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
© AstraZeneca 2020
-
PATIENT MEDICATION INFORMATION
Patient Information
KOSELUGO (ko-SEL-u-go)
(selumetinib) capsulesWhat is KOSELUGO?
KOSELUGO is a prescription medicine that is used to treat children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cannot be completely removed by surgery.
It is not known if KOSELUGO is safe and effective in children under 2 years of age.
Before taking KOSELUGO, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have eye problems.
-
are pregnant or plan to become pregnant. KOSELUGO can harm your unborn baby.
- ∘ Your healthcare provider should check to see if you are pregnant before you begin treatment with KOSELUGO.
- ∘ Females who are able to become pregnant should use effective birth control (contraception) during treatment with KOSELUGO and for 1 week after your last dose.
- ∘ Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with KOSELUGO and for 1 week after your last dose.
- ∘ Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with KOSELUGO.
-
are breastfeeding or plan to breastfeed. It is not known if KOSELUGO passes into your breast milk.
- ∘ Do not breastfeed during treatment with KOSELUGO and for 1 week after your last dose.
- ∘ Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. Especially tell your healthcare provider if you are taking aspirin, blood thinners, or other medicines to treat blood clots. KOSELUGO contains vitamin E which may increase your risk of bleeding.
How should I take KOSELUGO?
- Take KOSELUGO exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking KOSELUGO unless your healthcare provider tells you to.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with KOSELUGO if you have side effects.
- Your healthcare provider will decide on the right dose of KOSELUGO based on your weight or size (body surface area) and how many capsules of KOSELUGO to take.
- KOSELUGO should be taken around the same time each day, about 12 hours apart.
- Take KOSELUGO on an empty stomach. Do not eat food for 2 hours before your dose and 1 hour after your dose.
- Swallow KOSELUGO capsules whole with water. Do not chew, dissolve, or open the capsules.
- If you miss a dose of KOSELUGO, take it as soon as you remember. If it is less than 6 hours before your next scheduled dose, take your next dose at your regular time. Do not make up for the missed dose.
- If you vomit at any time after taking KOSELUGO, do not take an additional dose. Take your next dose at your regular time.
What should I avoid while taking KOSELUGO?
Do not drink grapefruit juice, eat grapefruit or take supplements that contain grapefruit or St. John’s Wort during treatment with KOSELUGO.
What are the possible side effects of KOSELUGO?
KOSELUGO may cause serious side effects, including:
-
Heart Problems. KOSELUGO can lower the amount of blood pumped by your heart which is common and can also be severe. Your healthcare provider will do tests before and during treatment with KOSELUGO to check how well your heart is working. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- persistent coughing or wheezing
- shortness of breath
- swelling of your ankles and feet
- tiredness
- increased heart rate
-
Eye Problems. KOSELUGO can cause eye problems that can lead to blindness. Your healthcare provider will check your vision before and during treatment with KOSELUGO. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- ∘ blurred vision
- ∘ loss of vision
- ∘ dark spots in your vision (floaters)
- ∘ other changes to your vision
- Severe diarrhea. Diarrhea is common with KOSELUGO and can also be severe. Tell your healthcare provider right away the first time that you get diarrhea during treatment with KOSELUGO. Your healthcare provider may give you medicine to help control your diarrhea and may tell you to drink more fluids.
-
Skin Rash. Skin rashes are common with KOSELUGO and can also be severe. Tell your healthcare provider if you get any of the following signs or symptoms:
- rash that covers a large area of your body
- peeling skin
- blisters
-
Muscle problems (rhabdomyolysis). Muscle problems are common with KOSELUGO and can also be severe. Treatment with KOSELUGO may increase the level of enzyme in your blood called creatine phosphokinase (CPK) and may be a sign of muscle damage. Your healthcare provider should do a blood test to check your blood levels of CPK before you start taking KOSELUGO and during treatment. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- muscle aches or pain
- muscle spasms and weakness
- dark, reddish urine
- Your healthcare provider may change your dose, temporarily stop, or permanently ask you to stop taking KOSELUGO if you have any of these side effects.
- The most common side effects of KOSELUGO are:
- vomiting
- stomach pain
- nausea
- dry skin
- feeling of tiredness, weakness or lacking energy
- muscle and bone pain
- fever
- inflammation of the mouth
- headache
- redness around the fingernails
- itching
These are not all the possible side effects of KOSELUGO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store KOSELUGO?
- Store KOSELUGO at room temperature, between 68°F to 77°F (20°C to 25°C).
- The bottle of KOSELUGO contains a desiccant packet to reduce moisture. Do not throw away desiccant packet.
- Keep KOSELUGO in its original bottle.
Keep KOSELUGO and all medicines out of the reach of children.
General information about the safe and effective use of KOSELUGO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KOSELUGO for a condition for which it was not prescribed. Do not give KOSELUGO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about KOSELUGO that is written for a healthcare professional.
What are the ingredients in KOSELUGO?
Active ingredient: selumetinib.
Inactive ingredients:
Capsule contains: vitamin E polyethylene glycol succinate.
The 10 mg capsule shell contains: hypromellose, carrageenan, potassium chloride, titanium dioxide, carnauba wax, and purified water.
The 10 mg capsule printing ink contains: shellac, iron oxide black, propylene glycol, and ammonium hydroxide.
The 25 mg capsule shell contains: hypromellose, carrageenan, potassium chloride, titanium dioxide, FD&C blue 2, ferric oxide yellow, purified water, carnauba wax and/or corn starch.
The 25 mg printing ink contains: ferric oxide red, ferric oxide yellow, FD&C Blue 2 aluminum lake, carnauba wax, shellac, glyceryl monooleate.
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2020
For more information, go to website www.KOSELUGO.com or call 1-800-236-9933
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: April/2020
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 10mg
NDC: 0310-0610-60
Koselugo™
(selumetinib)
capsules
10 mg
Rx only
Dispense in original bottle. Do not remove
desiccant. Protect from moisture.
60 capsules AstraZeneca
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 25mg
NDC: 0310-0625-60
Koselugo™
(selumetinib)
capsules
25 mg
Rx only
Dispense in original bottle.Do not remove
desiccant. Protect from moisture.
60 capsules AstraZeneca
-
INGREDIENTS AND APPEARANCE
KOSELUGO
selumetinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-0610 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELUMETINIB (UNII: 6UH91I579U) (SELUMETINIB - UNII:6UH91I579U) SELUMETINIB 10 mg Inactive Ingredients Ingredient Name Strength TOCOPHERSOLAN (UNII: O03S90U1F2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CARRAGEENAN (UNII: 5C69YCD2YJ) POTASSIUM CHLORIDE (UNII: 660YQ98I10) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) ISOPROPYL ALCOHOL (UNII: ND2M416302) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code SEL;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-0610-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213756 04/16/2020 KOSELUGO
selumetinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-0625 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELUMETINIB (UNII: 6UH91I579U) (SELUMETINIB - UNII:6UH91I579U) SELUMETINIB 25 mg Inactive Ingredients Ingredient Name Strength TOCOPHERSOLAN (UNII: O03S90U1F2) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CARRAGEENAN (UNII: 5C69YCD2YJ) POTASSIUM CHLORIDE (UNII: 660YQ98I10) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) SHELLAC (UNII: 46N107B71O) 1-BUTANOL, 4-(ETHENYLOXY)- (UNII: 8LGM5RZX0N) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) GLYCERYL OLEATE (UNII: 4PC054V79P) Product Characteristics Color BLUE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code SEL;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-0625-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213756 04/16/2020 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)
Trademark Results [KOSELUGO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KOSELUGO 88639031 not registered Live/Pending |
AstraZeneca AB 2019-10-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.