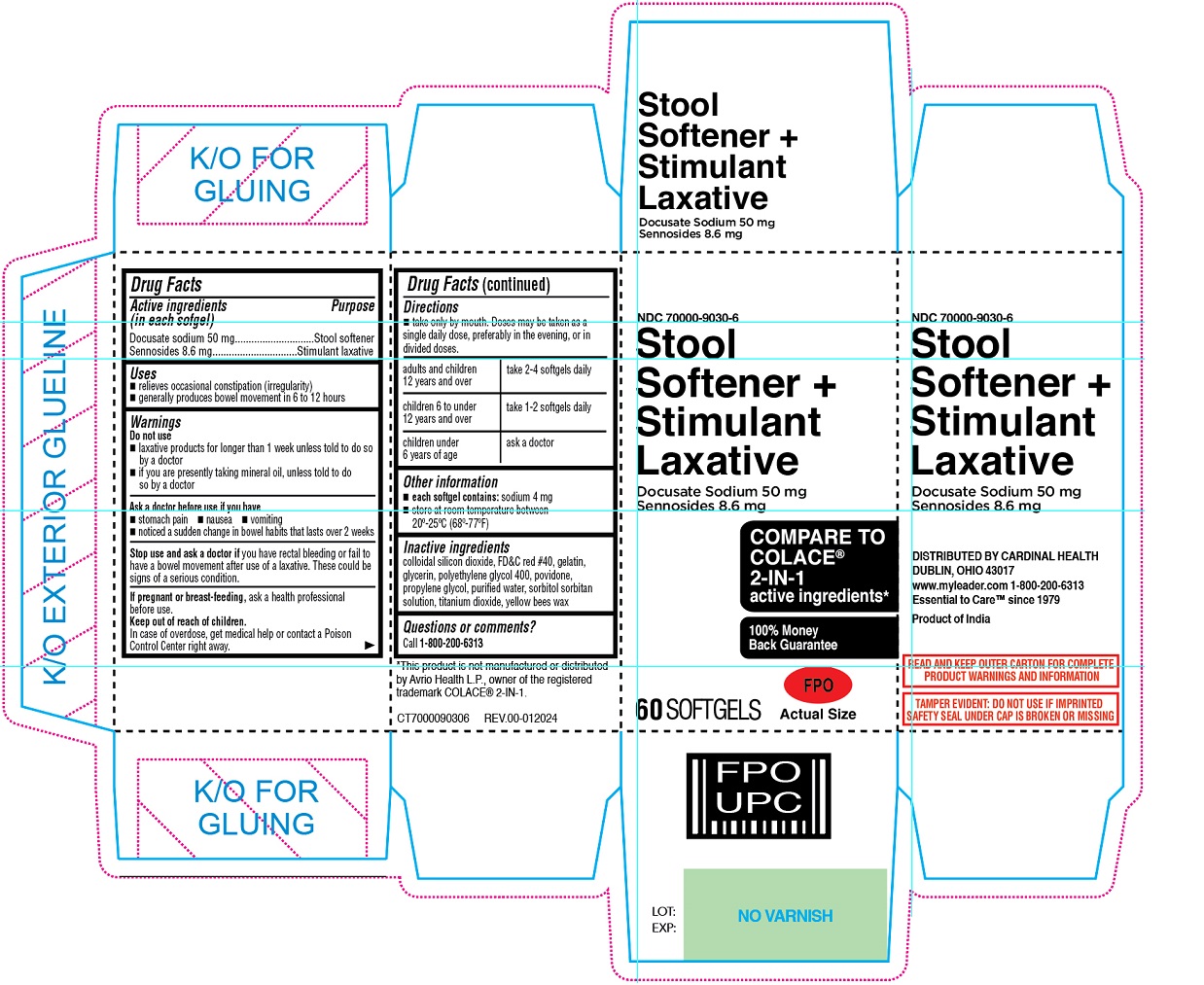
Stool Softener + Stimulant Laxative SOFTGEL
Leader Stool Softener Plus Stimulant Laxative SOFTGEL by
Drug Labeling and Warnings
Leader Stool Softener Plus Stimulant Laxative SOFTGEL by is a Otc medication manufactured, distributed, or labeled by Cardinal Health 110, LLC. DBA Leader. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEADER STOOL SOFTENER PLUS STIMULANT LAXATIVE SOFTGEL- docusate sodium, sennosides capsule, liquid filled
Cardinal Health 110, LLC. DBA Leader
----------
Stool Softener + Stimulant Laxative SOFTGEL
Uses
relieves occasional constipation (irregularity)
generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
laxative products for longer than 1 week unless told to do so by a doctor if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
stomach pain nausea vomiting noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
| adults and children 12 years and over | take 2-4 softgels daily |
| children 6 to under 12 years and over | take 1-2 softgels daily |
| children under 6 years of age | ask a doctor |
Other information
each softgel contains: sodium 4 mg
store at room temperature between 20°-25°C (68°-77°F)
Inactive ingredients
colloidal silicon dioxide, FD&C red #40, gelatin, glycerin, polyethylene glycol 400, povidone, propylene glycol, purified water, sorbitol sorbitan solution, titanium dioxide, yellow bees wax
COMPARE TO COLACE® 2-IN-1 active ingredients*
100% Money Back Guarantee
DISTRIBUTE BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www. myleader.com 1-800-200-6313
Essential to Care™ since 1979
Product of India
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT WARNINGS AND INFORMATION
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Avrio Health L.P., owner of the registered trademark COLACE® 2-IN-1.
| LEADER STOOL SOFTENER PLUS STIMULANT LAXATIVE SOFTGEL
docusate sodium, sennosides capsule, liquid filled |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Cardinal Health 110, LLC. DBA Leader (063997360) |