CVS PHARMACY ASSORTED BERRY ANTACID- calcium carbonate tablet, chewable
CVS PHARMACY by
Drug Labeling and Warnings
CVS PHARMACY by is a Otc medication manufactured, distributed, or labeled by CVS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
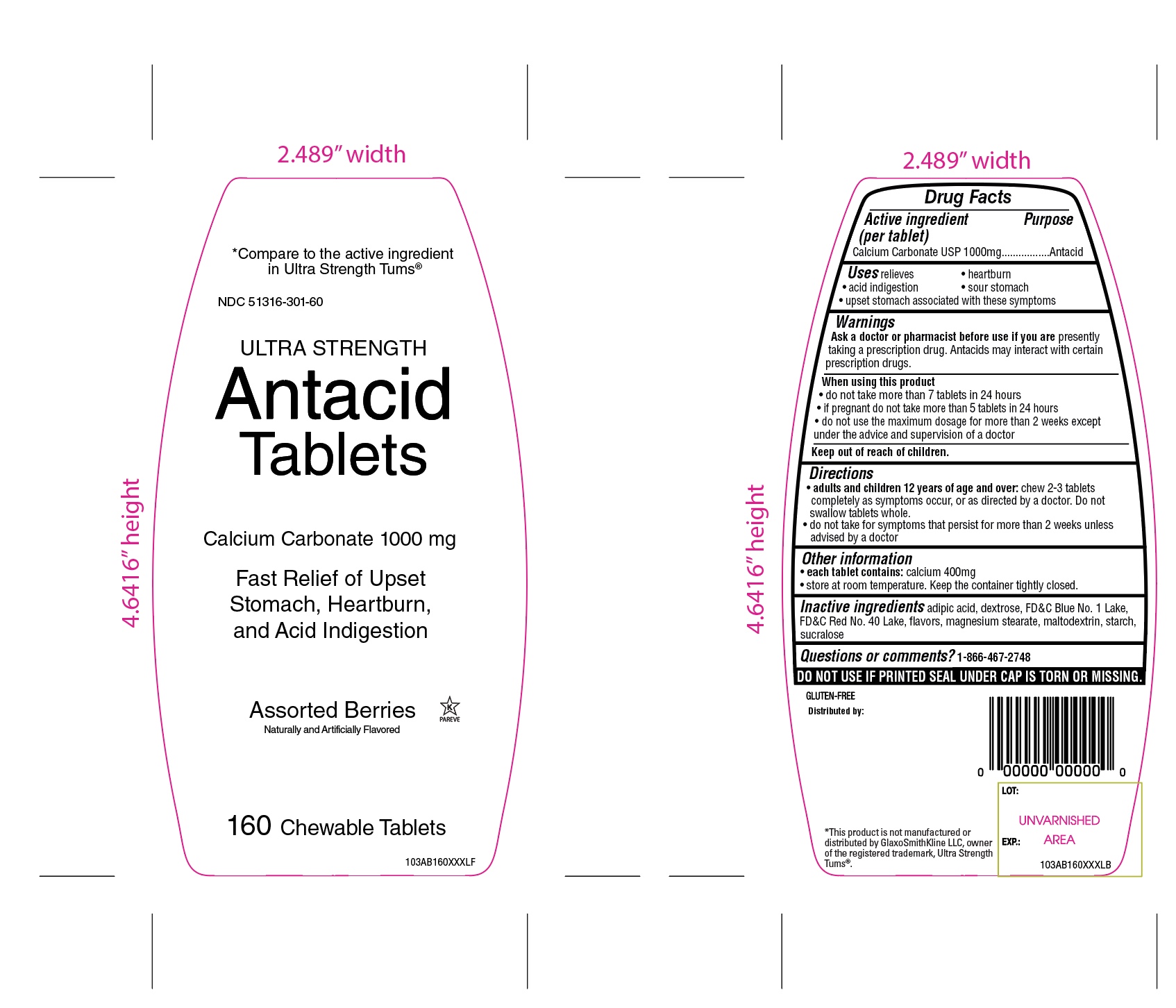
- Active ingredient (per tablet)
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- When using this product
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
NDC# 51316-301-72
*Compare to the active ingredient in Ultra Strength Tums ®
ULTRA STRENGTH
Antacid Tablets
Calcium Carbonate 1000 mg
Fast Relief of Upset Stomach, Heartburn, and Acid Indigestion
Assorted Berry
Naturally and Artificially Flavored
K PAREVE
GLUTEN FREE
72 Chewable Tablets
DISTRIBUTED
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Ultra Strength Tums ®
Package Label for 72 Chewable tablets
-
INGREDIENTS AND APPEARANCE
CVS PHARMACY ASSORTED BERRY ANTACID
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51316-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1000 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) ADIPIC ACID (UNII: 76A0JE0FKJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color pink Score no score Shape ROUND Size 19mm Flavor BERRY (Assorted) Imprint Code RP103 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51316-301-72 72 in 1 BOTTLE; Type 0: Not a Combination Product 08/02/2021 2 NDC: 51316-301-60 160 in 1 BOTTLE; Type 0: Not a Combination Product 08/02/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 08/02/2021 Labeler - CVS (062312574)
Trademark Results [CVS PHARMACY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CVS PHARMACY 97287242 not registered Live/Pending |
CVS Pharmacy, Inc. 2022-02-28 |
 CVS PHARMACY 87622772 5453318 Live/Registered |
CVS Pharmacy, Inc. 2017-09-26 |
 CVS PHARMACY 86893256 5031926 Live/Registered |
CVS Pharmacy, Inc. 2016-02-01 |
 CVS PHARMACY 86892916 5027198 Live/Registered |
CVS Pharmacy, Inc. 2016-02-01 |
 CVS PHARMACY 86882356 5157603 Live/Registered |
CVS Pharmacy, Inc. 2016-01-21 |
 CVS PHARMACY 86879078 5157590 Live/Registered |
CVS Pharmacy, Inc. 2016-01-19 |
 CVS PHARMACY 86123747 5022909 Live/Registered |
CVS Pharmacy, Inc. 2013-11-20 |
 CVS PHARMACY 86091649 4608526 Live/Registered |
CVS Pharmacy, Inc. 2013-10-15 |
 CVS PHARMACY 86064266 4591680 Live/Registered |
CVS Pharmacy, Inc. 2013-09-13 |
 CVS PHARMACY 86025887 4673785 Live/Registered |
CVS Pharmacy, Inc. 2013-08-01 |
 CVS PHARMACY 76372220 2774665 Live/Registered |
CVS PHARMACY, INC. 2002-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.