MYCOPHENOLATE MOFETIL capsule MYCOPHENOLATE MOFETIL tablet, film coated
Mycophenolate mofetil by
Drug Labeling and Warnings
Mycophenolate mofetil by is a Prescription medication manufactured, distributed, or labeled by American Health Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES AND SERIOUS INFECTIONS
Use during pregnancy is associated with increased risks of first trimester pregnancy loss and congenital malformations. Females of reproductive potential (FRP) must be counseled regarding pregnancy prevention and planning (see WARNINGS and PRECAUTIONS).
Immunosuppression may lead to increased susceptibility to infection and possible development of lymphoma. Only physicians experienced in immunosuppressive therapy and management of renal, cardiac or hepatic transplant patients should prescribe Mycophenolate mofetil. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient (see WARNINGS and PRECAUTIONS).
-
DESCRIPTION
Mycophenolate mofetil is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has an empirical formula of C 23H 31NO 7, a molecular weight of 433.50, and the following structural formula:
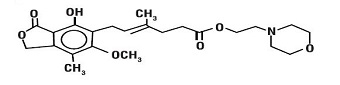
Mycophenolate mofetil is a white to off-white crystalline powder. It is slightly soluble in water (43 µg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for mycophenolate mofetil are 5.6 for the morpholino group and 8.5 for the phenolic group.
Mycophenolate mofetil hydrochloride has a solubility of 65.8 mg/mL in 5% Dextrose Injection USP (D5W). The pH of the reconstituted solution is 2.4 to 4.1.
Mycophenolate mofetil is available for oral administration as capsules containing 250 mg of mycophenolate mofetil, and tablets containing 500 mg of mycophenolate mofetil.
Inactive ingredients in mycophenolate mofetil capsules, USP 250 mg include croscarmellose sodium, magnesium stearate, povidone (K-30), microcrystalline cellulose. The capsule shells contain yellow iron oxide, FD&C red # 3, gelatin, sodium lauryl sulfate, and titanium dioxide.
Inactive ingredients in mycophenolate mofetil tablets, USP 500 mg include microcrystalline cellulose, croscarmellose sodium, povidone [K-30], magnesium stearate (Vegetable) and opadry brown.
The opadry brown contains FD & C blue #1 aluminum lake, FD & C red #40 aluminum lake, hypromellose, iron oxide red, polyethylene glycol and titanium dioxide.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Mycophenolate mofetil has been demonstrated in experimental animal models to prolong the survival of allogeneic transplants (kidney, heart, liver, intestine, limb, small bowel, pancreatic islets, and bone marrow).
Mycophenolate mofetil has also been shown to reverse ongoing acute rejection in the canine renal and rat cardiac allograft models. Mycophenolate mofetil also inhibited proliferative arteriopathy in experimental models of aortic and cardiac allografts in rats, as well as in primate cardiac xenografts. Mycophenolate mofetil was used alone or in combination with other immunosuppressive agents in these studies. Mycophenolate mofetil has been demonstrated to inhibit immunologically mediated inflammatory responses in animal models and to inhibit tumor development and prolong survival in murine tumor transplant models.
Mycophenolate mofetil is rapidly absorbed following oral administration and hydrolyzed to form MPA, which is the active metabolite. MPA is a potent, selective, uncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), and therefore inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation into DNA. Because T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, whereas other cell types can utilize salvage pathways, MPA has potent cytostatic effects on lymphocytes. MPA inhibits proliferative responses of T- and B-lymphocytes to both mitogenic and allospecific stimulation. Addition of guanosine or deoxyguanosine reverses the cytostatic effects of MPA on lymphocytes. MPA also suppresses antibody formation by B-lymphocytes. MPA prevents the glycosylation of lymphocyte and monocyte glycoproteins that are involved in intercellular adhesion to endothelial cells and may inhibit recruitment of leukocytes into sites of inflammation and graft rejection. Mycophenolate mofetil did not inhibit early events in the activation of human peripheral blood mononuclear cells, such as the production of interleukin-1 (IL-1) and interleukin-2 (IL-2), but did block the coupling of these events to DNA synthesis and proliferation.
Pharmacokinetics
Following oral and intravenous administration, mycophenolate mofetil undergoes rapid and complete metabolism to MPA, the active metabolite. Oral absorption of the drug is rapid and essentially complete. MPA is metabolized to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. The parent drug, mycophenolate mofetil, can be measured systemically during the intravenous infusion; however, shortly (about 5 minutes) after the infusion is stopped or after oral administration, MMF concentration is below the limit of quantitation (0.4 mcg/mL).
Absorption
In 12 healthy volunteers, the mean absolute bioavailability of oral mycophenolate mofetil relative to intravenous mycophenolate mofetil (based on MPA AUC) was 94%. The area under the plasma-concentration time curve (AUC) for MPA appears to increase in a dose-proportional fashion in renal transplant patients receiving multiple doses of mycophenolate mofetil up to a daily dose of 3 g (see Table 1).
Food (27 g fat, 650 calories) had no effect on the extent of absorption (MPA AUC) of mycophenolate mofetil when administered at doses of 1.5 g bid to renal transplant patients. However, MPA C max was decreased by 40% in the presence of food (see DOSAGE AND ADMINISTRATION).
Distribution
The mean (±SD) apparent volume of distribution of MPA in 12 healthy volunteers is approximately 3.6 (±1.5) and 4.0 (±1.2) L/kg following intravenous and oral administration, respectively. MPA, at clinically relevant concentrations, is 97% bound to plasma albumin. MPAG is 82% bound to plasma albumin at MPAG concentration ranges that are normally seen in stable renal transplant patients; however, at higher MPAG concentrations (observed in patients with renal impairment or delayed renal graft function), the binding of MPA may be reduced as a result of competition between MPAG and MPA for protein binding. Mean blood to plasma ratio of radioactivity concentrations was approximately 0.6 indicating that MPA and MPAG do not extensively distribute into the cellular fractions of blood.
In vitro studies to evaluate the effect of other agents on the binding of MPA to human serum albumin (HSA) or plasma proteins showed that salicylate (at 25 mg/dL with HSA) and MPAG (at ≥ 460 mcg/mL with plasma proteins) increased the free fraction of MPA. At concentrations that exceeded what is encountered clinically, cyclosporine, digoxin, naproxen, prednisone, propranolol, tacrolimus, theophylline, tolbutamide, and warfarin did not increase the free fraction of MPA. MPA at concentrations as high as 100 mcg/mL had little effect on the binding of warfarin, digoxin or propranolol, but decreased the binding of theophylline from 53% to 45% and phenytoin from 90% to 87%.
Metabolism
Following oral and intravenous dosing, mycophenolate mofetil undergoes complete metabolism to MPA, the active metabolite. Metabolism to MPA occurs presystemically after oral dosing. MPA is metabolized principally by glucuronyl transferase to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. In vivo, MPAG is converted to MPA via enterohepatic recirculation. The following metabolites of the 2-hydroxyethyl-morpholino moiety are also recovered in the urine following oral administration of mycophenolate mofetil to healthy subjects: N-(2-carboxymethyl)-morpholine, N-(2-hydroxyethyl)-morpholine, and the N-oxide of N-(2-hydroxyethyl)-morpholine.
Secondary peaks in the plasma MPA concentration-time profile are usually observed 6 to 12 hours postdose. The coadministration of cholestyramine (4 g tid) resulted in approximately a 40% decrease in the MPA AUC (largely as a consequence of lower concentrations in the terminal portion of the profile). These observations suggest that enterohepatic recirculation contributes to MPA plasma concentrations.
Increased plasma concentrations of mycophenolate mofetil metabolites (MPA 50% increase and MPAG about a 3-fold to 6-fold increase) are observed in patients with renal insufficiency (see CLINICAL PHARMACOLOGY: Special Populations).
Excretion
Negligible amount of drug is excreted as MPA (< 1% of dose) in the urine. Orally administered radiolabeled mycophenolate mofetil resulted in complete recovery of the administered dose, with 93% of the administered dose recovered in the urine and 6% recovered in feces. Most (about 87%) of the administered dose is excreted in the urine as MPAG. At clinically encountered concentrations, MPA and MPAG are usually not removed by hemodialysis. However, at high MPAG plasma concentrations (> 100 mcg/mL), small amounts of MPAG are removed. Bile acid sequestrants, such as cholestyramine, reduce MPA AUC by interfering with enterohepatic circulation of the drug (see OVERDOSAGE).
Mean (±SD) apparent half-life and plasma clearance of MPA are 17.9 (±6.5) hours and 193 (±48) mL/min following oral administration and 16.6 (±5.8) hours and 177 (±31) mL/min following intravenous administration, respectively.
Pharmacokinetics in Healthy Volunteers, Renal, Cardiac, and Hepatic Transplant Patients
Shown below are the mean (±SD) pharmacokinetic parameters for MPA following the administration of mycophenolate mofetil given as single doses to healthy volunteers and multiple doses to renal, cardiac, and hepatic transplant patients. In the early posttransplant period (< 40 days posttransplant), renal, cardiac, and hepatic transplant patients had mean MPA AUCs approximately 20% to 41% lower and mean C max approximately 32% to 44% lower compared to the late transplant period (3 to 6 months posttransplant).Mean MPA AUC values following administration of 1 g bid intravenous mycophenolate mofetil over 2 hours to renal transplant patients for 5 days were about 24% higher than those observed after oral administration of a similar dose in the immediate posttransplant phase. In hepatic transplant patients, administration of 1 g bid intravenous mycophenolate mofetil followed by 1.5 g bid oral mycophenolate mofetil resulted in mean MPA AUC values similar to those found in renal transplant patients administered 1 g mycophenolate mofetil bid.
Table 1 Pharmacokinetic Parameters for MPA [mean (±SD)] Following Administration of Mycophenolate Mofetil to Healthy Volunteers (Single Dose), Renal, Cardiac, and Hepatic Transplant Patients (Multiple Doses) - * AUC(0-12h) values quoted are extrapolated from data from samples collected over 4 hours.
Dose/Route
T max
(h)
C max
(µg/mL)
Total AUC
(µg∙h/mL)
Healthy Volunteers
(single dose)1 g/oral
0.80 (±0.36)
(n=129)24.5 (±9.5)
(n=129)63.9 (±16.2)
(n=117)Renal Transplant Patients (bid dosing)
Time After Transplantation
Dose/Route
T max
(h)
C max
(µg/mL)
Interdosing Interval
AUC (0–12h)
(µg∙h/mL)
5 days
1 g/iv
1.58 (±0.46)
(n=31)
12.0 (±3.82)
(n=31)
40.8 (±11.4)
(n=31)
6 days
1 g/oral
1.33 (±1.05)
(n=31)
10.7 (±4.83)
(n=31)
32.9 (±15)
(n=31)
Early (< 40 days)
1 g/oral
1.31 (±0.76)
(n=25)
8.16 (±4.50)
(n=25)
27.3 (±10.9)
(n=25)
Early (< 40 days)
1.5 g/oral
1.21 (±0.81)
(n=27)
13.5 (±8.18)
(n=27)
38.4 (±15.4)
(n=27)
Late (>3 months)
1.5 g/oral
0.90 (±0.24)
(n=23)
24.1 (±12.1)
(n=23)
65.3 (±35.4)
(n=23)
Cardiac Transplant Patients (bid dosing)
Time After Transplantation
Dose/Route
T max
(h)
C max
(µg/mL)
Interdosing Interval
AUC (0–12h)
(µg∙h/mL)
Early (Day before discharge)
1.5 g/oral
1.8 (±1.3)
(n=11)
11.5 (±6.8)
(n=11)
43.3 (±20.8)
(n=9)
Late (> 6 months)
1.5 g/oral
1.1 (±0.7)
(n=52)
20.0 (±9.4)
(n=52)
54.1 * (±20.4)
(n=49)
Hepatic Transplant Patients (bid dosing)
Time After Transplantation
Dose/Route
T max
(h)
C max
(µg/mL)
Interdosing Interval
AUC (0–12h)
(µg∙h/mL)
4 to 9 days
1 g/iv
1.50 (±0.517)
(n=22)
17.0 (±12.7)
(n=22)
34.0 (±17.4)
(n=22)
Early (5 to 8 days)
1.5 g/oral
1.15 (±0.432)
(n=20)
13.1 (±6.76)
(n=20)
29.2 (±11.9)
(n=20)
Late (> 6 months)
1.5 g/oral
1.54 (±0.51)
(n=6)
19.3 (±11.7)
(n=6)
49.3 (±14.8)
(n=6)
Two 500 mg tablets have been shown to be bioequivalent to four 250 mg capsules. Five mL of the 200 mg/mL constituted oral suspension have been shown to be bioequivalent to four 250 mg capsules.
Special Populations
Shown below are the mean (±SD) pharmacokinetic parameters for MPA following the administration of oral mycophenolate mofetil given as single doses to non-transplant subjects with renal or hepatic impairment.
Table 2 Pharmacokinetic Parameters for MPA [mean (±SD)] Following Single Doses of Mycophenolate Mofetil Capsules in Chronic Renal and Hepatic Impairment Renal Impairment
(no. of patients)Dose
T max
(h)
C max
(µg/mL)
AUC (0–96h)
(µg∙h/mL)
Healthy Volunteers
GFR > 80 mL/min/1.73 m 2
(n=6)
1 g
0.75 (±0.27)
25.3 (±7.99)
45 (±22.6)
Mild Renal Impairment
GFR 50 to 80 mL/min/1.73 m 2
(n=6)
1 g
0.75 (±0.27)
26 (±3.82)
59.9 (±12.9)
Moderate Renal Impairment
GFR 25 to 49 mL/min/1.73 m 2
(n=6)
1 g
0.75 (±0.27)
19 (±13.2)
52.9 (±25.5)
Severe Renal Impairment
GFR < 25 mL/min/1.73 m 2
(n=7)
1 g
1 (±0.41)
16.3 (±10.8)
78.6 (±46.4)
Hepatic Impairment
(no. of patients)Dose
T max
(h)
C max
(µg/mL)
AUC (0–48h)
(µg∙h/mL)
Healthy Volunteers (n=6)
1 g
0.63 (±0.14)
24.3 (±5.73)
29 (±5.78)
Alcoholic Cirrhosis (n=18)
1 g
0.85 (±0.58)
22.4 (±10.1)
29.8 (±10.7)
Renal Insufficiency
In a single-dose study, MMF was administered as capsule or intravenous infusion over 40 minutes. Plasma MPA AUC observed after oral dosing to volunteers with severe chronic renal impairment [glomerular filtration rate (GFR) < 25 mL/min/1.73 m 2] was about 75% higher relative to that observed in healthy volunteers (GFR > 80 mL/min/1.73 m 2). In addition, the single-dose plasma MPAG AUC was 3-fold to 6-fold higher in volunteers with severe renal impairment than in volunteers with mild renal impairment or healthy volunteers, consistent with the known renal elimination of MPAG. No data are available on the safety of long-term exposure to this level of MPAG.
Plasma MPA AUC observed after single-dose (1 g) intravenous dosing to volunteers (n=4) with severe chronic renal impairment (GFR < 25 mL/min/1.73 m 2) was 62.4 µgh/mL (±19.3). Multiple dosing of mycophenolate mofetil in patients with severe chronic renal impairment has not been studied (see PRECAUTIONS: Patients with Renal Impairment and DOSAGE AND ADMINISTRATION).
In patients with delayed renal graft function posttransplant, mean MPA AUC (0-12h) was comparable to that seen in posttransplant patients without delayed renal graft function. There is a potential for a transient increase in the free fraction and concentration of plasma MPA in patients with delayed renal graft function. However, dose adjustment does not appear to be necessary in patients with delayed renal graft function. Mean plasma MPAG AUC (0-12h) was 2-fold to 3-fold higher than in posttransplant patients without delayed renal graft function (see PRECAUTIONS: Patients with Renal Impairment and DOSAGE AND ADMINISTRATION).
In 8 patients with primary graft non-function following renal transplantation, plasma concentrations of MPAG accumulated about 6-fold to 8-fold after multiple dosing for 28 days. Accumulation of MPA was about 1-fold to 2-fold.
The pharmacokinetics of mycophenolate mofetil are not altered by hemodialysis. Hemodialysis usually does not remove MPA or MPAG. At high concentrations of MPAG (> 100 µg/mL), hemodialysis removes only small amounts of MPAG.
Hepatic Insufficiency
In a single-dose (1 g oral) study of 18 volunteers with alcoholic cirrhosis and 6 healthy volunteers, hepatic MPA glucuronidation processes appeared to be relatively unaffected by hepatic parenchymal disease when pharmacokinetic parameters of healthy volunteers and alcoholic cirrhosis patients within this study were compared. However, it should be noted that for unexplained reasons, the healthy volunteers in this study had about a 50% lower AUC as compared to healthy volunteers in other studies, thus making comparisons between volunteers with alcoholic cirrhosis and healthy volunteers difficult. Effects of hepatic disease on this process probably depend on the particular disease. Hepatic disease with other etiologies, such as primary biliary cirrhosis, may show a different effect. In a single-dose (1 g intravenous) study of 6 volunteers with severe hepatic impairment (aminopyrine breath test less than 0.2% of dose) due to alcoholic cirrhosis, MMF was rapidly converted to MPA. MPA AUC was 44.1 µg∙h/mL (±15.5).
Pediatrics
The pharmacokinetic parameters of MPA and MPAG have been evaluated in 55 pediatric patients (ranging from 1 year to 18 years of age) receiving mycophenolate mofetil oral suspension at a dose of 600 mg/m 2 bid (up to a maximum of 1 g bid) after allogeneic renal transplantation. The pharmacokinetic data for MPA is provided in Table 3.
Table 3 Mean (±SD) Computed Pharmacokinetic Parameters for MPA by Age and Time After Allogeneic Renal Transplantation - * adjusted to a dose of 600 mg/m2
- † a subset of 1 to < 6 yr
- ‡ n=20
- § n=16
Age Group
(n)
Time
T max
(h)
Dose Adjusted*
C max (µg/mL)
Dose Adjusted*
AUC (0–12h) (µg∙h/mL)
1 to < 2 yr (6) †
Early (Day 7)
3.03 (4.70)
10.3 (5.80)
22.5 (6.66)
1 to < 6 yr (17)
1.63 (2.85)
13.2 (7.16)
27.4 (9.54)
6 to < 12 yr (16)
0.940 (0.546)
13.1 (6.30)
33.2 (12.1)
12 to 18 yr (21)
1.16 (0.830)
11.7 (10.7)
26.3 (9.14) ‡
1 to < 2 yr (4) †
Late (Month 3)
0.725 (0.276)
23.8 (13.4)
47.4 (14.7)
1 to < 6 yr (15)
0.989 (0.511)
22.7 (10.1)
49.7 (18.2)
6 to < 12 yr (14)
1.21 (0.532)
27.8 (14.3)
61.9 (19.6)
12 to 18 yr (17)
0.978 (0.484)
17.9 (9.57)
53.6 (20.3) §
1 to < 2 yr (4) †
Late (Month 9)
0.604 (0.208)
25.6 (4.25)
55.8 (11.6)
1 to < 6 yr (12)
0.869 (0.479)
30.4 (9.16)
61.0 (10.7)
6 to < 12 yr (11)
1.12 (0.462)
29.2 (12.6)
66.8 (21.2)
12 to 18 yr (14)
1.09 (0.518)
18.1 (7.29)
56.7 (14)
The Mycophenolate Mofetil oral suspension dose of 600 mg/m 2 bid (up to a maximum of 1 g bid) achieved mean MPA AUC values in pediatric patients similar to those seen in adult renal transplant patients receiving Mycophenolate Mofetil Capsules at a dose of 1 g bid in the early posttransplant period. There was wide variability in the data. As observed in adults, early posttransplant MPA AUC values were approximately 45% to 53% lower than those observed in the later posttransplant period (> 3 months). MPA AUC values were similar in the early and late posttransplant period across the 1 year to 18 year age range.
Gender
Data obtained from several studies were pooled to look at any gender-related differences in the pharmacokinetics of MPA (data were adjusted to 1 g oral dose). Mean (±SD) MPA AUC (0-12h) for males (n=79) was 32.0 (±14.5) and for females (n=41) was 36.5 (±18.8) mcgh/mL while mean (±SD) MPA C max was 9.96 (±6.19) in the males and 10.6 (±5.64) mcg/mL in the females. These differences are not of clinical significance.
-
CLINICAL STUDIES
Adults
The safety and efficacy of mycophenolate mofetil in combination with corticosteroids and cyclosporine for the prevention of organ rejection were assessed in randomized, double-blind, multicenter trials in renal (3 trials), in cardiac (1 trial), and in hepatic (1 trial) adult transplant patients.
Renal Transplant
Adults
The three renal studies compared two dose levels of oral mycophenolate mofetil (1 g bid and 1.5 g bid) with azathioprine (2 studies) or placebo (1 study) when administered in combination with cyclosporine (Sandimmune ®) and corticosteroids to prevent acute rejection episodes. One study also included antithymocyte globulin (ATGAM ®) induction therapy. These studies are described by geographic location of the investigational sites. One study was conducted in the USA at 14 sites, one study was conducted in Europe at 20 sites, and one study was conducted in Europe, Canada, and Australia at a total of 21 sites.
The primary efficacy endpoint was the proportion of patients in each treatment group who experienced treatment failure within the first 6 months after transplantation (defined as biopsy-proven acute rejection on treatment or the occurrence of death, graft loss or early termination from the study for any reason without prior biopsy-proven rejection). Mycophenolate mofetil, when administered with antithymocyte globulin (ATGAM ®) induction (one study) and with cyclosporine and corticosteroids (all three studies), was compared to the following three therapeutic regimens: (1) antithymocyte globulin (ATGAM ®) induction/azathioprine/cyclosporine/corticosteroids, (2) azathioprine/cyclosporine/corticosteroids, and (3) cyclosporine/corticosteroids.
Mycophenolate mofetil, in combination with corticosteroids and cyclosporine reduced (statistically significant at 0.05 level) the incidence of treatment failure within the first 6 months following transplantation. Table 4 and Table 5 summarize the results of these studies. These tables show (1) the proportion of patients experiencing treatment failure, (2) the proportion of patients who experienced biopsy-proven acute rejection on treatment, and (3) early termination, for any reason other than graft loss or death, without a prior biopsy-proven acute rejection episode. Patients who prematurely discontinued treatment were followed for the occurrence of death or graft loss, and the cumulative incidence of graft loss and patient death are summarized separately. Patients who prematurely discontinued treatment were not followed for the occurrence of acute rejection after termination. More patients receiving mycophenolate mofetil discontinued without prior biopsy-proven rejection, death or graft loss than discontinued in the control groups, with the highest rate in the mycophenolate mofetil 3 g/day group. Therefore, the acute rejection rates may be underestimates, particularly in the mycophenolate mofetil 3 g/day group.
Table 4 Renal Transplant Studies Incidence of Treatment Failure (Biopsy-proven Rejection or Early Termination for Any Reason) - * Antithymocyte globulin induction/MMF or azathioprine/cyclosporine/corticosteroids.
- † Does not include death and graft loss as reason for early termination.
- ‡ MMF or azathioprine/cyclosporine/corticosteroids.
- § MMF or placebo/cyclosporine/corticosteroids.
USA Study*
(N=499 patients)Mycophenolate mofetil
2 g/day
(n=167 patients)Mycophenolate mofetil
3 g/day
(n=166 patients)Azathioprine
1 to 2 mg/kg/day
(n=166 patients)All treatment failures
31.1%
31.3%
47.6%
Early termination without prior acute rejection †
9.6%
12.7%
6.0%
Biopsy-proven rejection episode on treatment
19.8%
17.5%
38%
Europe/Canada/ Australia Study‡
(N=503 patients)Mycophenolate mofetil
2 g/day
(n=173 patients)Mycophenolate mofetil
3 g/day
(n=164 patients)Azathioprine
100 to 150 mg/kg/day
(n=166 patients)All treatment failures
38.2%
34.8%
50.0%
Early termination without prior acute rejection †
13.9%
15.2%
10.2%
Biopsy-proven rejection episode on treatment
19.7%
15.9%
35.5%
Europe Study§
(N=491 patients)Mycophenolate mofetil
2 g/day
(n=165 patients)Mycophenolate mofetil
3 g/day
(n=160 patients)Placebo
(n=166 patients)All treatment failures
30.3%
38.8%
56.0%
Early termination without prior acute rejection †
11.5%
22.5%
7.2%
Biopsy-proven rejection episode on treatment
17.0%
13.8%
46.4%
The cumulative incidence of 12-month graft loss or patient death is presented below. No advantage of mycophenolate mofetil with respect to graft loss or patient death was established. Numerically, patients receiving mycophenolate mofetil 2 g/day and 3 g/day experienced a better outcome than controls in all three studies; patients receiving mycophenolate mofetil 2 g/day experienced a better outcome than mycophenolate mofetil 3 g/day in two of the three studies. Patients in all treatment groups who terminated treatment early were found to have a poor outcome with respect to graft loss or patient death at 1 year.
Table 5 Renal Transplant Studies Cumulative Incidence of Combined Graft Loss or Patient Death at 12 Months Study
Mycophenolate mofetil
2 g/dayMycophenolate mofetil
3 g/dayControl
(Azathioprine or Placebo)USA
8.5%
11.5%
12.2%
Europe/Canada/Australia
11.7%
11.0%
13.6%
Europe
8.5%
10.0%
11.5%
Pediatrics
One open-label, safety and pharmacokinetic study of mycophenolate mofetil oral suspension 600 mg/m 2 bid (up to 1 g bid) in combination with cyclosporine and corticosteroids was performed at centers in the US (9), Europe (5) and Australia (1) in 100 pediatric patients (3 months to 18 years of age) for the prevention of renal allograft rejection. Mycophenolate mofetil was well tolerated in pediatric patients (see ADVERSE REACTIONS), and the pharmacokinetics profile was similar to that seen in adult patients dosed with 1 g bid mycophenolate mofetil capsules (see CLINICAL PHARMACOLOGY: Pharmacokinetics). The rate of biopsy-proven rejection was similar across the age groups (3 months to < 6 years, 6 years to < 12 years, 12 years to 18 years). The overall biopsy-proven rejection rate at 6 months was comparable to adults. The combined incidence of graft loss (5%) and patient death (2%) at 12 months posttransplant was similar to that observed in adult renal transplant patients.
Cardiac Transplant
A double-blind, randomized, comparative, parallel-group, multicenter study in primary cardiac transplant recipients was performed at 20 centers in the United States, 1 in Canada, 5 in Europe and 2 in Australia. The total number of patients enrolled was 650; 72 never received study drug and 578 received study drug. Patients received mycophenolate mofetil 1.5 g bid (n=289) or azathioprine 1.5 to 3 mg/kg/day (n=289), in combination with cyclosporine (Sandimmune ® or Neoral ®) and corticosteroids as maintenance immunosuppressive therapy. The two primary efficacy endpoints were: (1) the proportion of patients who, after transplantation, had at least one endomyocardial biopsy-proven rejection with hemodynamic compromise, or were retransplanted or died, within the first 6 months, and (2) the proportion of patients who died or were retransplanted during the first 12 months following transplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection for up to 6 months and for the occurrence of death for 1 year.
- Rejection: No difference was established between mycophenolate mofetil and azathioprine (AZA) with respect to biopsy-proven rejection with hemodynamic compromise.
- Survival: Mycophenolate mofetil was shown to be at least as effective as AZA in preventing death or retransplantation at 1 year (see Table 6).
Table 6 Rejection at 6 Months/Death or Retransplantation at 1 Year - * Hemodynamic compromise occurred if any of the following criteria were met: pulmonary capillary wedge pressure ≥ 20 mm or a 25% increase; cardiac index < 2.0 L/min/m2 or a 25% decrease; ejection fraction ≤ 30%; pulmonary artery oxygen saturation ≤ 60% or a 25% decrease; presence of new S3 gallop; fractional shortening was ≤ 20% or a 25% decrease; inotropic support required to manage the clinical condition.
All Patients
Treated Patients
AZA
N = 323Mycophenolate
mofetil
N = 327AZA
N = 289Mycophenolate
mofetil
N = 289Biopsy-proven rejection with hemodynamic compromise at 6 months *
121 (38%)
120 (37%)
100 (35%)
92 (32%)
Death or retransplantation at 1 year
49 (15.2%)
42 (12.8%)
33 (11.4%)
18 (6.2%)
Hepatic Transplant
A double-blind, randomized, comparative, parallel-group, multicenter study in primary hepatic transplant recipients was performed at 16 centers in the United States, 2 in Canada, 4 in Europe and 1 in Australia. The total number of patients enrolled was 565. Per protocol, patients received mycophenolate mofetil 1 g bid intravenously for up to 14 days followed by mycophenolate mofetil 1.5 g bid orally or azathioprine 1 to 2 mg/kg/day intravenously followed by azathioprine 1 to 2 mg/kg/day orally, in combination with cyclosporine (Neoral ®) and corticosteroids as maintenance immunosuppressive therapy. The actual median oral dose of azathioprine on study was 1.5 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) initially and 1.26 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) at 12 months. The two primary endpoints were: (1) the proportion of patients who experienced, in the first 6 months posttransplantation, one or more episodes of biopsy-proven and treated rejection or death or retransplantation, and (2) the proportion of patients who experienced graft loss (death or retransplantation) during the first 12 months posttransplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection and for the occurrence of graft loss (death or retransplantation) for 1 year.
Results
In combination with corticosteroids and cyclosporine, mycophenolate mofetil obtained a lower rate of acute rejection at 6 months and a similar rate of death or retransplantation at 1 year compared to azathioprine.
Table 7 Rejection at 6 Months/Death or Retransplantation at 1 Year AZA
N = 287Mycophenolate mofetil
N = 278Biopsy-proven, treated rejection at 6 months (includes death or retransplantation)
137 (47.7%)
107 (38.5%)
Death or retransplantation at 1 year
42 (14.6%)
41 (14.7%)
-
INDICATIONS AND USAGE
Renal, Cardiac, and Hepatic Transplant
Mycophenolate mofetil is indicated for the prophylaxis of organ rejection in patients receiving allogeneic renal, cardiac or hepatic transplants. Mycophenolate mofetil should be used concomitantly with cyclosporine and corticosteroids.
Mycophenolate mofetil Intravenous is an alternative dosage form to mycophenolate mofetil tablets, capsules and oral suspension. Mycophenolate mofetil Intravenous should be administered within 24 hours following transplantation. Mycophenolate mofetil Intravenous can be administered for up to 14 days; patients should be switched to oral mycophenolate mofetil as soon as they can tolerate oral medication.
-
CONTRAINDICATIONS
Allergic reactions to mycophenolate mofetil have been observed; therefore, mycophenolate mofetil is contraindicated in patients with a hypersensitivity to mycophenolate mofetil, mycophenolic acid or any component of the drug product. Mycophenolate mofetil Intravenous is contraindicated in patients who are allergic to Polysorbate 80 (TWEEN).
-
WARNINGS
(see boxed WARNING)
Embryofetal Toxicity
Mycophenolate mofetil (MMF) can cause fetal harm when administered to a pregnant female. Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system (see PRECAUTIONS: Pregnancy).
Pregnancy Exposure Prevention and Planning
Females of reproductive potential must be made aware of the increased risk of first trimester pregnancy loss and congenital malformations and must be counseled regarding pregnancy prevention and planning. For recommended pregnancy testing and contraception methods (see PRECAUTIONS: Pregnancy Exposure Prevention and Planning).
Lymphoma and Malignancy
Patients receiving immunosuppressive regimens involving combinations of drugs, including mycophenolate mofetil, as part of an immunosuppressive regimen are at increased risk of developing lymphomas and other malignancies, particularly of the skin (see ADVERSE REACTIONS).The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Lymphoproliferative disease or lymphoma developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g) with other immunosuppressive agents in controlled clinical trials of renal, cardiac, and hepatic transplant patients (see ADVERSE REACTIONS).
In pediatric patients, no other malignancies besides lymphoproliferative disorder (2/148 patients) have been observed (see ADVERSE REACTIONS).
Combination with Other Immunosuppressive Agents
Mycophenolate mofetil has been administered in combination with the following agents in clinical trials: antithymocyte globulin (ATGAM ®), OKT3 (Orthoclone OKT ® 3), cyclosporine (Sandimmune ®, Neoral ®) and corticosteroids. The efficacy and safety of the use of mycophenolate mofetil in combination with other immunosuppressive agents have not been determined.
Serious Infections
Patients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing bacterial, fungal, protozoal and new or reactivated viral infections, including opportunistic infections. These infections may lead to serious, including fatal outcomes. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution (see ADVERSE REACTIONS).
New or Reactivated Viral Infections
Polyomavirus associated nephropathy (PVAN), JC virus associated progressive multifocal leukoencephalopathy (PML), cytomegalovirus (CMV) infections, reactivation of hepatitis B (HBV) or hepatitis C (HCV) have been reported in patients treated with immunosuppressants, including Mycophenolate Mofetil. Reduction in immunosuppression should be considered for patients who develop evidence of new or reactivated viral infections. Physicians should also consider the risk that reduced immunosuppression represents to the functioning allograft.
PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss (see ADVERSE REACTIONS: Postmarketing Experience). Patient monitoring may help detect patients at risk for PVAN.
PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function (see ADVERSE REACTIONS: Postmarketing Experience). In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease.
Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
Neutropenia
Severe neutropenia [absolute neutrophil count (ANC) < 0.5 x 10 3/µL] developed in up to 2% of renal, up to 2.8% of cardiac, and up to 3.6% of hepatic transplant patients receiving mycophenolate mofetil 3 g daily (see ADVERSE REACTIONS). Patients receiving mycophenolate mofetil should be monitored for neutropenia (see PRECAUTIONS: Laboratory Tests).The development of neutropenia may be related to mycophenolate mofetil itself, concomitant medications, viral infections, or some combination of these causes. If neutropenia develops (ANC < 1.3 x 10 3/µL), dosing with mycophenolate mofetil should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately (see DOSAGE AND ADMINISTRATION). Neutropenia has been observed most frequently in the period from 31 to 180 days posttransplant in patients treated for prevention of renal, cardiac, and hepatic rejection.
Patients receiving mycophenolate mofetil should be instructed to report immediately any evidence of infection, unexpected bruising, bleeding or any other manifestation of bone marrow depression.
Pure Red Cell Aplasia (PRCA)
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with mycophenolate mofetil in combination with other immunosuppressive agents. The mechanism for mycophenolate mofetil induced PRCA is unknown; the relative contribution of other immunosuppressants and their combinations in an immunosuppression regimen are also unknown. In some cases, PRCA was found to be reversible with dose reduction or cessation of mycophenolate mofetil therapy. In transplant patients, however, reduced immunosuppression may place the graft at risk.
CAUTION: MYCOPHENOLATE MOFETIL INTRAVENOUS SOLUTION MUST NOT BE ADMINISTERED BY RAPID OR BOLUS INTRAVENOUS INJECTION.
-
PRECAUTIONS
Pregnancy Exposure Prevention and Planning
Females of reproductive potential must be made aware of the increased risk of first trimester pregnancy loss and congenital malformations and must be counseled regarding pregnancy prevention and planning.
Females of reproductive potential include girls who have entered puberty and all women who have a uterus and have not passed through menopause. Menopause is the permanent end of menstruation and fertility. Menopause should be clinically confirmed by a patient’s healthcare practitioner. Some commonly used diagnostic criteria include 1) 12 months of spontaneous amenorrhea (not amenorrhea induced by a medical condition or medical therapy) or 2) postsurgical from a bilateral oophorectomy.
Pregnancy Testing
To prevent unplanned exposure during pregnancy, females of reproductive potential should have a serum or urine pregnancy test with a sensitivity of at least 25 mIU/mL immediately before starting mycophenolate mofetil. Another pregnancy test with the same sensitivity should be done 8 to 10 days later. Repeat pregnancy tests should be performed during routine follow-up visits. Results of all pregnancy tests should be discussed with the patient.
In the event of a positive pregnancy test, females should be counseled with regard to whether the maternal benefits of mycophenolate treatment may outweigh the risks to the fetus in certain situations.
Contraception
Females of reproductive potential taking mycophenolate mofetil must receive contraceptive counseling and use acceptable contraception (see Table 8 for acceptable contraception methods). Patients must use acceptable birth control during entire mycophenolate mofetil therapy, and for 6 weeks after stopping mycophenolate mofetil, unless the patient chooses abstinence (she chooses to avoid heterosexual intercourse completely).
Patients should be aware that mycophenolate mofetil reduces blood levels of the hormones in the oral contraceptive pill and could theoretically reduce its effectiveness (see PRECAUTIONS: Information for Patients and PRECAUTIONS: Drug Interactions: Oral Contraceptives).
Table 8 Acceptable Contraception Methods for Females of Reproductive Potential Pick from the following birth control options: Option 1
Methods to Use Alone
- Intrauterine devices (IUDs)
- Tubal sterilization
- Patient’s partner had a vasectomy
OR
Option 2
Hormone Methods
choose 1Barrier Methods
choose 1Choose One Hormone Method
AND
One Barrier MethodEstrogen and Progesterone
- Oral Contraceptive Pill
- Transdermal patch
- Vaginal ring
Progesterone-only
- Injection
- Implant
AND
- Diaphragm with spermicide
- Cervical cap with spermicide
- Contraceptive sponge
- Male condom
- Female condom
OR
Option 3
Barrier Methods
choose 1Barrier Methods
choose 1Choose One
Barrier Method from each column
(must choose two methods)
- Diaphragm with spermicide
- Cervical cap with spermicide
- Contraceptive sponge
AND
- Male condom
- Female condom
Pregnancy Planning
For patients who are considering pregnancy, consider alternative immunosuppressants with less potential for embryofetal toxicity. Risks and benefits of Mycophenolate mofetil should be discussed with the patient.
Gastrointestinal Disorders
Gastrointestinal bleeding (requiring hospitalization) has been observed in approximately 3% of renal, in 1.7% of cardiac, and in 5.4% of hepatic transplant patients treated with Mycophenolate mofetil 3 g daily. In pediatric renal transplant patients, 5/148 cases of gastrointestinal bleeding (requiring hospitalization) were observed.
Gastrointestinal perforations have rarely been observed. Most patients receiving Mycophenolate mofetil were also receiving other drugs known to be associated with these complications. Patients with active peptic ulcer disease were excluded from enrollment in studies with mycophenolate mofetil. Because Mycophenolate mofetil has been associated with an increased incidence of digestive system adverse events, including infrequent cases of gastrointestinal tract ulceration, hemorrhage, and perforation, Mycophenolate mofetil should be administered with caution in patients with active serious digestive system disease.
Patients with Renal Impairment
Subjects with severe chronic renal impairment (GFR < 25 mL/min/1.73 m 2) who have received single doses of Mycophenolate mofetil showed higher plasma MPA and MPAG AUCs relative to subjects with lesser degrees of renal impairment or normal healthy volunteers. No data are available on the safety of long-term exposure to these levels of MPAG. Doses of Mycophenolate mofetil greater than 1 g administered twice a day to renal transplant patients should be avoided and they should be carefully observed (see CLINICAL PHARMACOLOGY: Pharmacokinetics and DOSAGE AND ADMINISTRATION).
No data are available for cardiac or hepatic transplant patients with severe chronic renal impairment. Mycophenolate mofetil may be used for cardiac or hepatic transplant patients with severe chronic renal impairment if the potential benefits outweigh the potential risks.
In patients with delayed renal graft function posttransplant, mean MPA AUC (0-12h) was comparable, but MPAG AUC (0-12h) was 2-fold to 3-fold higher, compared to that seen in posttransplant patients without delayed renal graft function. In the three controlled studies of prevention of renal rejection, there were 298 of 1483 patients (20%) with delayed graft function. Although patients with delayed graft function have a higher incidence of certain adverse events (anemia, thrombocytopenia, hyperkalemia) than patients without delayed graft function, these events were not more frequent in patients receiving mycophenolate mofetil than azathioprine or placebo. No dose adjustment is recommended for these patients; however, they should be carefully observed (see CLINICAL PHARMACOLOGY: Pharmacokinetics and DOSAGE AND ADMINISTRATION).
Infections in Cardiac Transplant Patients
In cardiac transplant patients, the overall incidence of opportunistic infections was approximately 10% higher in patients treated with Mycophenolate mofetil than in those receiving azathioprine therapy, but this difference was not associated with excess mortality due to infection/sepsis among patients treated with Mycophenolate mofetil (see ADVERSE REACTIONS).
There were more herpes virus (H. simplex, H. zoster, and cytomegalovirus) infections in cardiac transplant patients treated with Mycophenolate mofetil compared to those treated with azathioprine (see ADVERSE REACTIONS).
Concomitant Medications
It is recommended that Mycophenolate mofetil not be administered concomitantly with azathioprine because both have the potential to cause bone marrow suppression and such concomitant administration has not been studied clinically.
In view of the significant reduction in the AUC of MPA by cholestyramine, caution should be used in the concomitant administration of Mycophenolate mofetil with drugs that interfere with enterohepatic recirculation because of the potential to reduce the efficacy of Mycophenolate mofetil (see PRECAUTIONS: Drug Interactions).
Patients with HGPRT Deficiency
Mycophenolate mofetil is an IMPDH (inosine monophosphate dehydrogenase) inhibitor; therefore it should be avoided in patients with rare hereditary deficiency of hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) such as Lesch-Nyhan and Kelley-Seegmiller syndrome.
Immunizations
During treatment with Mycophenolate mofetil, the use of live attenuated vaccines should be avoided and patients should be advised that vaccinations may be less effective (see PRECAUTIONS: Drug Interactions: Live Vaccines).
Information for Patients
See Medication Guide
- Inform females of reproductive potential that use of mycophenolate mofetil during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, and advise them as to the appropriate steps to manage these risks, including that they must use acceptable contraception (see WARNINGS: Embryofetal Toxicity, PRECAUTIONS: Pregnancy Exposure Prevention and Planning).
- Discuss pregnancy testing, pregnancy prevention and planning with females of reproductive potential. In the event of a positive pregnancy test, females should be counseled with regard to whether the maternal benefits of mycophenolate treatment may outweigh the risks to the fetus in certain situations.
- Females of reproductive potential must use acceptable birth control during entire mycophenolate mofetil therapy and for 6 weeks after stopping mycophenolate mofetil, unless the patient chooses to avoid heterosexual intercourse completely (abstinence) (see PRECAUTIONS: Pregnancy Exposure Prevention and Planning,Table 8).
- For patients who are considering pregnancy, discuss appropriate alternative immunosuppressants with less potential for embryofetal toxicity. Risks and benefits of mycophenolate mofetil should be discussed with the patient.
- Give patients complete dosage instructions and inform them about the increased risk of lymphoproliferative disease and certain other malignancies.
- Inform patients that they need repeated appropriate laboratory tests while they are taking mycophenolate mofetil.
- Advise patients that they should not breastfeed during mycophenolate mofetil therapy.
Laboratory Tests
Complete blood counts should be performed weekly during the first month, twice monthly for the second and third months of treatment, then monthly through the first year (see WARNINGS, ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
Drug Interactions
Drug interaction studies with mycophenolate mofetil have been conducted with acyclovir, antacids, cholestyramine, cyclosporine, ganciclovir, oral contraceptives, sevelamer, trimethoprim/sulfamethoxazole, norfloxacin and metronidazole. Drug interaction studies have not been conducted with other drugs that may be commonly administered to renal, cardiac or hepatic transplant patients. Mycophenolate mofetil has not been administered concomitantly with azathioprine.
Acyclovir
Coadministration of mycophenolate mofetil (1 g) and acyclovir (800 mg) to 12 healthy volunteers resulted in no significant change in MPA AUC and C max. However, MPAG and acyclovir plasma AUCs were increased 10.6% and 21.9%, respectively. Because MPAG plasma concentrations are increased in the presence of renal impairment, as are acyclovir concentrations, the potential exists for mycophenolate and acyclovir or its prodrug (e.g., valacyclovir) to compete for tubular secretion, further increasing the concentrations of both drugs.
Antacids with Magnesium and Aluminum Hydroxides
Absorption of a single dose of mycophenolate mofetil (2 g) was decreased when administered to ten rheumatoid arthritis patients also taking Maalox ® TC (10 mL qid). The C max and AUC (0-24h) for MPA were 33% and 17% lower, respectively, than when mycophenolate mofetil was administered alone under fasting conditions. Mycophenolate mofetil may be administered to patients who are also taking antacids containing magnesium and aluminum hydroxides; however, it is recommended that Mycophenolate mofetil and the antacid not be administered simultaneously.
Proton Pump Inhibitors (PPIs)
Coadministration of PPIs (e.g., lansoprazole, pantoprazole) in single doses to healthy volunteers and multiple doses to transplant patients receiving Mycophenolate mofetil has been reported to reduce the exposure to mycophenolic acid (MPA). An approximate reduction of 30 to 70% in the C max and 25% to 35% in the AUC of MPA has been observed, possibly due to a decrease in MPA solubility at an increased gastric pH. The clinical impact of reduced MPA exposure on organ rejection has not been established in transplant patients receiving PPIs and Mycophenolate mofetil. Because clinical relevance has not been established, PPIs should be used with caution when coadministered to transplant patients being treated with Mycophenolate mofetil.
Cholestyramine
Following single-dose administration of 1.5 g mycophenolate mofetil to 12 healthy volunteers pretreated with 4 g tid of cholestyramine for 4 days, MPA AUC decreased approximately 40%. This decrease is consistent with interruption of enterohepatic recirculation which may be due to binding of recirculating MPAG with cholestyramine in the intestine. Some degree of enterohepatic recirculation is also anticipated following intravenous administration of mycophenolate mofetil. Therefore, mycophenolate mofetil is not recommended to be given with cholestyramine or other agents that may interfere with enterohepatic recirculation.
Cyclosporine
Cyclosporine (Sandimmune ®) pharmacokinetics (at doses of 275 to 415 mg/day) were unaffected by single and multiple doses of 1.5 g bid of mycophenolate mofetil in 10 stable renal transplant patients. The mean (±SD) AUC (0-12h) and C max of cyclosporine after 14 days of multiple doses of mycophenolate mofetil were 3290 (±822) ngh/mL and 753 (±161) ng/mL, respectively, compared to 3245 (±1088) ngh/mL and 700 (±246) ng/mL, respectively, 1 week before administration of mycophenolate mofetil.
Cyclosporine A interferes with MPA enterohepatic recirculation. In renal transplant patients, mean MPA exposure (AUC 0-12h) was approximately 30 to 50% greater when mycophenolate mofetil is administered without cyclosporine compared with when mycophenolate mofetil is coadministered with cyclosporine. This interaction is due to cyclosporine inhibition of multidrug-resistance-associated protein 2 (MRP-2) transporter in the biliary tract, thereby preventing the excretion of MPAG into the bile that would lead to enterohepatic recirculation of MPA. This information should be taken into consideration when MMF is used without cyclosporine; changes in MPA exposure should be expected when switching patients from cyclosporine A to one of the immunosuppressants which do not interfere with MPA's enterohepatic cycle (e.g. tacrolimus; belatacept).
Telmisartan
Concomitant administration of telmisartan and mycophenolate Mofetil resulted in an approximately 30% decrease in mycophenolic acid (MPA) concentrations. Telmisartan changes MPA’s elimination by enhancing PPAR gamma (peroxisome proliferator-activated receptor gamma) expression, which in turn results in an enhanced UGT1A9 expression and activity.
Ganciclovir
Following single-dose administration to 12 stable renal transplant patients, no pharmacokinetic interaction was observed between mycophenolate mofetil (1.5 g) and intravenous ganciclovir (5 mg/kg). Mean (±SD) ganciclovir AUC and C max (n=10) were 54.3 (±19.0) µgh/mL and 11.5 (±1.8) µg/mL, respectively, after coadministration of the two drugs, compared to 51.0 (±17) µgh/mL and 10.6 (±2.0) µg/mL, respectively, after administration of intravenous ganciclovir alone. The mean (±SD) AUC and C max of MPA (n=12) after coadministration were 80.9 (±21.6) µgh/mL and 27.8 (±13.9) µg/mL, respectively, compared to values of 80.3 (±16.4) µgh/mL and 30.9 (±11.2) µg/mL, respectively, after administration of mycophenolate mofetil alone. Because MPAG plasma concentrations are increased in the presence of renal impairment, as are ganciclovir concentrations, the two drugs will compete for tubular secretion and thus further increases in concentrations of both drugs may occur. In patients with renal impairment in which MMF and ganciclovir or its prodrug (e.g., valganciclovir) are coadministered, patients should be monitored carefully.
Oral Contraceptives
A study of coadministration of mycophenolate mofetil (1 g bid) and combined oral contraceptives containing ethinylestradiol (0.02 mg to 0.04 mg) and levonorgestrel (0.05 mg to 0.20 mg), desogestrel (0.15 mg) or gestodene (0.05 mg to 0.10 mg) was conducted in 18 women with psoriasis over 3 consecutive menstrual cycles. Mean AUC (0-24h) was similar for ethinylestradiol and 3-keto desogestrel; however, mean levonorgestrel AUC (0-24h) significantly decreased by about 15%. There was large inter-patient variability (%CV in the range of 60% to 70%) in the data, especially for ethinylestradiol. Mean serum levels of LH, FSH and progesterone were not significantly affected. Mycophenolate mofetil may not have any influence on the ovulation-suppressing action of the studied oral contraceptives. It is recommended to coadminister mycophenolate mofetil with hormonal contraceptives (e.g., birth control pill, transdermal patch, vaginal ring, injection, and implant) with caution and additional barrier contraceptive methods must be used (see PRECAUTIONS: Pregnancy Exposure Prevention and Planning).
Sevelamer
Concomitant administration of sevelamer and mycophenolate mofetil in adult and pediatric patients decreased the mean MPA C max and AUC 0-12h by 36% and 26% respectively. This data suggest that sevelamer and other calcium free phosphate binders should not be administered simultaneously with mycophenolate mofetil. Alternatively, it is recommended that sevelamer and other calcium free phosphate binders preferentially could be given 2 hours after mycophenolate mofetil intake to minimize the impact on the absorption of MPA.
Trimethoprim/sulfamethoxazole
Following single-dose administration of mycophenolate mofetil (1.5 g) to 12 healthy male volunteers on day 8 of a 10 day course of trimethoprim 160 mg/sulfamethoxazole 800 mg administered bid, no effect on the bioavailability of MPA was observed. The mean (±SD) AUC and C max of MPA after concomitant administration were 75.2 (±19.8) µgh/mL and 34.0 (±6.6) µg/mL, respectively, compared to 79.2 (±27.9) µgh/mL and 34.2 (±10.7) µg/mL, respectively, after administration of mycophenolate mofetil alone.
Norfloxacin and Metronidazole
Following single-dose administration of mycophenolate mofetil (1 g) to 11 healthy volunteers on day 4 of a 5 day course of a combination of norfloxacin and metronidazole, the mean MPA AUC 0-48h was significantly reduced by 33% compared to the administration of mycophenolate mofetil alone (p<0.05). Therefore, mycophenolate mofetil is not recommended to be given with the combination of norfloxacin and metronidazole. There was no significant effect on mean MPA AUC 0-48h when mycophenolate mofetil was concomitantly administered with norfloxacin or metronidazole separately. The mean (±SD) MPA AUC 0-48h after coadministration of mycophenolate mofetil with norfloxacin or metronidazole separately was 48.3 (±24) μg·h/mL and 42.7 (±23) µg·h/mL, respectively, compared with 56.2 (±24) µg·h/mL after administration of mycophenolate mofetil alone.
Ciprofloxacin and Amoxicillin plus Clavulanic Acid
A total of 64 mycophenolate mofetil-treated renal transplant recipients received either oral ciprofloxacin 500 mg bid or amoxicillin plus clavulanic acid 375 mg tid for 7 or at least 14 days. Approximately 50% reductions in median trough MPA concentrations (predose) from baseline (mycophenolate alone) were observed in 3 days following commencement of oral ciprofloxacin or amoxicillin plus clavulanic acid. These reductions in trough MPA concentrations tended to diminish within 14 days of antibiotic therapy and ceased within 3 days after discontinuation of antibiotics. The postulated mechanism for this interaction is an antibiotic-induced reduction in glucuronidase-possessing enteric organisms leading to a decrease in enterohepatic recirculation of MPA. The change in trough level may not accurately represent changes in overall MPA exposure; therefore, clinical relevance of these observations is unclear.
Rifampin
In a single heart-lung transplant patient, after correction for dose, a 67% decrease in MPA exposure (AUC 0-12h) has been observed with concomitant administration of mycophenolate mofetil and rifampin. Therefore, mycophenolate mofetil is not recommended to be given with rifampin concomitantly unless the benefit outweighs the risk.
Other Interactions
The measured value for renal clearance of MPAG indicates removal occurs by renal tubular secretion as well as glomerular filtration. Consistent with this, coadministration of probenecid, a known inhibitor of tubular secretion, with mycophenolate mofetil in monkeys results in a 3-fold increase in plasma MPAG AUC and a 2-fold increase in plasma MPA AUC. Thus, other drugs known to undergo renal tubular secretion may compete with MPAG and thereby raise plasma concentrations of MPAG or the other drug undergoing tubular secretion.
Drugs that alter the gastrointestinal flora may interact with mycophenolate mofetil by disrupting enterohepatic recirculation. Interference of MPAG hydrolysis may lead to less MPA available for absorption.
Live Vaccines
During treatment with mycophenolate mofetil, the use of live attenuated vaccines should be avoided and patients should be advised that vaccinations may be less effective (see PRECAUTIONS: Immunizations).Influenza vaccination may be of value. Prescribers should refer to national guidelines for influenza vaccination.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week oral carcinogenicity study in mice, mycophenolate mofetil in daily doses up to 180 mg/kg was not tumorigenic. The highest dose tested was 0.5 times the recommended clinical dose (2 g/day) in renal transplant patients and 0.3 times the recommended clinical dose (3 g/day) in cardiac transplant patients when corrected for differences in body surface area (BSA). In a 104-week oral carcinogenicity study in rats, mycophenolate mofetil in daily doses up to 15 mg/kg was not tumorigenic. The highest dose was 0.08 times the recommended clinical dose in renal transplant patients and 0.05 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. While these animal doses were lower than those given to patients, they were maximal in those species and were considered adequate to evaluate the potential for human risk (see WARNINGS).
The genotoxic potential of mycophenolate mofetil was determined in five assays. Mycophenolate mofetil was genotoxic in the mouse lymphoma/thymidine kinase assay and the in vivo mouse micronucleus assay. Mycophenolate mofetil was not genotoxic in the bacterial mutation assay, the yeast mitotic gene conversion assay or the Chinese hamster ovary cell chromosomal aberration assay.
Mycophenolate mofetil had no effect on fertility of male rats at oral doses up to 20 mg/kg/day. This dose represents 0.1 times the recommended clinical dose in renal transplant patients and 0.07 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. In a female fertility and reproduction study conducted in rats, oral doses of 4.5 mg/kg/day caused malformations (principally of the head and eyes) in the first generation offspring in the absence of maternal toxicity. This dose was 0.02 times the recommended clinical dose in renal transplant patients and 0.01 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. No effects on fertility or reproductive parameters were evident in the dams or in the subsequent generation.
Pregnancy
Pregnancy Category D
See WARNINGS section.
Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system. In animal studies, congenital malformations and pregnancy loss occurred when pregnant rats and rabbits received mycophenolic acid at dose multiples similar to and less than clinical doses. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Risks and benefits of mycophenolate mofetil should be discussed with the patient. When appropriate, consider alternative immunosuppressants with less potential for embryofetal toxicity. In certain situations, the patient and her healthcare practitioner may decide that the maternal benefits outweigh the risks to the fetus. For those females using mycophenolate mofetil at any time during pregnancy and those becoming pregnant within 6 weeks of discontinuing therapy, the healthcare practitioner should report the pregnancy to the Mycophenolate Pregnancy Registry (1-800-617-8191). The healthcare practitioner should strongly encourage the patient to enroll in the pregnancy registry. The information provided to the registry will help the healthcare community better understand the effects of mycophenolate in pregnancy.
In the National Transplantation Pregnancy Registry (NTPR), there were data on 33 MMF-exposed pregnancies in 24 transplant patients; there were 15 spontaneous abortions (45%) and 18 live-born infants. Four of these 18 infants had structural malformations (22%). In postmarketing data (collected 1995-2007) on 77 females exposed to systemic MMF during pregnancy, 25 had spontaneous abortions and 14 had a malformed infant or fetus. Six of 14 malformed offspring had ear abnormalities. Because these postmarketing data are reported voluntarily, it is not always possible to reliably estimate the frequency of particular adverse outcomes. These malformations are similar to findings in animal reproductive toxicology studies. For comparison, the background rate for congenital anomalies in the United States is about 3%, and NTPR data show a rate of 4 - 5% among babies born to organ transplant patients using other immunosuppressive drugs.
In animal reproductive toxicology studies, there were increased rates of fetal resorptions and malformations in the absence of maternal toxicity. Female rats and rabbits received mycophenolate mofetil (MMF) doses equivalent to 0.02 to 0.9 times the recommended human dose for renal and cardiac transplant patients, based on body surface area conversions. In rat offspring, malformations included anophthalmia, agnathia, and hydrocephaly. In rabbit offspring, malformations included ectopia cordis, ectopic kidneys, diaphragmatic hernia, and umbilical hernia.
Nursing Mothers
Studies in rats treated with mycophenolate mofetil have shown mycophenolic acid to be excreted in milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from mycophenolate mofetil, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Based on pharmacokinetic and safety data in pediatric patients after renal transplantation, the recommended dose of mycophenolate mofetil oral suspension is 600 mg/m 2 bid (up to a maximum of 1 g bid). Also see CLINICAL PHARMACOLOGY, CLINICAL STUDIES, ADVERSE REACTIONS, and DOSAGE AND ADMINISTRATION.
Safety and effectiveness in pediatric patients receiving allogenic cardiac or hepatic transplants have not been established.
Geriatric Use
Clinical studies of mycophenolate mofetil did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant or other drug therapy. Elderly patients may be at an increased risk of adverse reactions compared with younger individuals (see ADVERSE REACTIONS).
-
ADVERSE REACTIONS
The principal adverse reactions associated with the administration of mycophenolate mofetil include diarrhea, leukopenia, sepsis, vomiting, and there is evidence of a higher frequency of certain types of infections e.g., opportunistic infection (see WARNINGS: Serious Infections and WARNINGS: New or Reactivated Viral Infections). The adverse event profile associated with the administration of mycophenolate mofetil Intravenous has been shown to be similar to that observed after administration of oral dosage forms of mycophenolate mofetil.
Mycophenolate Mofetil Oral
The incidence of adverse events for mycophenolate mofetil was determined in randomized, comparative, double-blind trials in prevention of rejection in renal (2 active, 1 placebo-controlled trials), cardiac (1 active-controlled trial), and hepatic (1 active-controlled trial) transplant patients.
Geriatrics
Elderly patients (≥ 65 years), particularly those who are receiving mycophenolate mofetil as part of a combination immunosuppressive regimen, may be at increased risk of certain infections (including cytomegalovirus [CMV] tissue invasive disease) and possibly gastrointestinal hemorrhage and pulmonary edema, compared to younger individuals (see PRECAUTIONS).
Safety data are summarized below for all active-controlled trials in renal (2 trials), cardiac (1 trial), and hepatic (1 trial) transplant patients. Approximately 53% of the renal patients, 65% of the cardiac patients, and 48% of the hepatic patients have been treated for more than 1 year. Adverse events reported in ≥ 20% of patients in the mycophenolate mofetil treatment groups are presented below.
Table 9 Adverse Events in Controlled Studies in Prevention of Renal, Cardiac or Hepatic Allograft Rejection (Reported in ≥ 20% of Patients in the Mycophenolate Mofetil Group) Renal Studies
Cardiac Study
Hepatic Study
Mycophenolate mofetil
2 g/dayMycophenolate mofetil
3 g/dayAzathioprine
1 to 2 mg/kg/day or 100 to 150 mg/dayMycophenolate mofetil
3 g/dayAzathioprine
1.5 to 3
mg/kg/dayMycophenolate mofetil
3 g/dayAzathioprine
1 to 2
mg/kg/day(n=336)
(n=330)
(n=326)
(n=289)
(n=289)
(n=277)
(n=287)
%
%
%
%
%
%
%
Body as a Whole
Pain
33.0
31.2
32.2
75.8
74.7
74.0
77.7
Abdominal pain
24.7
27.6
23.0
33.9
33.2
62.5
51.2
Fever
21.4
23.3
23.3
47.4
46.4
52.3
56.1
Headache
21.1
16.1
21.2
54.3
51.9
53.8
49.1
Infection
18.2
20.9
19.9
25.6
19.4
27.1
25.1
Sepsis
–
–
–
–
–
27.4
26.5
Asthenia
–
–
–
43.3
36.3
35.4
33.8
Chest pain
–
–
–
26.3
26.0
–
–
Back pain
–
–
–
34.6
28.4
46.6
47.4
Ascites
–
–
–
–
–
24.2
22.6
Hematologic and Lymphatic
Anemia
25.6
25.8
23.6
42.9
43.9
43.0
53.0
Leukopenia
23.2
34.5
24.8
30.4
39.1
45.8
39.0
Thrombocytopenia
–
–
–
23.5
27.0
38.3
42.2
Hypochromic anemia
–
–
–
24.6
23.5
–
–
Leukocytosis
–
–
–
40.5
35.6
22.4
21.3
Urogenital
Urinary tract infection
37.2
37
33.7
–
–
–
–
Kidney function abnormal
–
–
–
21.8
26.3
25.6
28.9
Cardiovascular
Hypertension
32.4
28.2
32.2
77.5
72.3
62.1
59.6
Hypotension
–
–
–
32.5
36.0
–
–
Cardiovascular disorder
–
–
–
25.6
24.2
–
–
Tachycardia
–
–
–
20.1
18.0
22.0
15.7
Metabolic and Nutritional
Peripheral edema
28.6
27.0
28.2
64.0
53.3
48.4
47.7
Hyper-cholesteremia
–
–
–
41.2
38.4
–
–
Edema
–
–
–
26.6
25.6
28.2
28.2
Hypokalemia
–
–
–
31.8
25.6
37.2
41.1
Hyperkalemia
–
–
–
–
–
22.0
23.7
Hyperglycemia
–
–
–
46.7
52.6
43.7
48.8
Creatinine increased
–
–
–
39.4
36.0
–
–
BUN increased
–
–
–
34.6
32.5
–
–
Lactic dehydrogenase increased
–
–
–
23.2
17.0
–
–
Hypomagnesemia
–
–
–
–
–
39.0
37.6
Hypocalcemia
–
–
–
–
–
30.0
30.0
Digestive
Diarrhea
31.0
36.1
20.9
45.3
34.3
51.3
49.8
Constipation
22.9
18.5
22.4
41.2
37.7
37.9
38.3
Nausea
19.9
23.6
24.5
54.0
54.3
54.5
51.2
Dyspepsia
–
–
–
–
–
22.4
20.9
Vomiting
–
–
–
33.9
28.4
32.9
33.4
Anorexia
–
–
–
–
–
25.3
17.1
Liver function tests abnormal
–
–
–
–
–
24.9
19.2
Respiratory
Infection
22.0
23.9
19.6
37.0
35.3
–
–
Dyspnea
–
–
–
36.7
36.3
31.0
30.3
Cough increased
–
–
–
31.1
25.6
–
–
Lung disorder
–
–
–
30.1
29.1
22.0
18.8
Sinusitis
–
–
–
26.0
19.0
–
–
Pleural effusion
–
–
–
–
–
34.3
35.9
Skin and Appendages
Rash
–
–
–
22.1
18.0
–
–
Nervous System
Tremor
–
–
–
24.2
23.9
33.9
35.5
Insomnia
–
–
–
40.8
37.7
52.3
47.0
Dizziness
–
–
–
28.7
27.7
–
–
Anxiety
–
–
–
28.4
23.9
–
–
Paresthesia
–
–
–
20.8
18.0
–
–
The placebo-controlled renal transplant study generally showed fewer adverse events occurring in ≥ 20% of patients. In addition, those that occurred were not only qualitatively similar to the azathioprine-controlled renal transplant studies, but also occurred at lower rates, particularly for infection, leukopenia, hypertension, diarrhea and respiratory infection.
The above data demonstrate that in three controlled trials for prevention of renal rejection, patients receiving 2 g/day of mycophenolate mofetil had an overall better safety profile than did patients receiving 3 g/day of mycophenolate mofetil.
The above data demonstrate that the types of adverse events observed in multicenter controlled trials in renal, cardiac, and hepatic transplant patients are qualitatively similar except for those that are unique to the specific organ involved.
Sepsis, which was generally CMV viremia, was slightly more common in renal transplant patients treated with mycophenolate mofetil compared to patients treated with azathioprine. The incidence of sepsis was comparable in mycophenolate mofetil and in azathioprine-treated patients in cardiac and hepatic studies.
In the digestive system, diarrhea was increased in renal and cardiac transplant patients receiving mycophenolate mofetil compared to patients receiving azathioprine, but was comparable in hepatic transplant patients treated with mycophenolate mofetil or azathioprine.
Patients receiving mycophenolate mofetil alone or as part of an immunosuppressive regimen are at increased risk of developing lymphomas and other malignancies, particularly of the skin (see WARNINGS: Lymphoma and Malignancy). The incidence of malignancies among the 1483 patients treated in controlled trials for the prevention of renal allograft rejection who were followed for ≥ 1 year was similar to the incidence reported in the literature for renal allograft recipients.
Lymphoproliferative disease or lymphoma developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g daily) with other immunosuppressive agents in controlled clinical trials of renal, cardiac, and hepatic transplant patients followed for at least 1 year (see WARNINGS: Lymphoma and Malignancy). Non-melanoma skin carcinomas occurred in 1.6% to 4.2% of patients, other types of malignancy in 0.7% to 2.1% of patients. Three-year safety data in renal and cardiac transplant patients did not reveal any unexpected changes in incidence of malignancy compared to the 1-year data.
In pediatric patients, no other malignancies besides lymphoproliferative disorder (2/148 patients) have been observed.
Severe neutropenia (ANC < 0.5 x 10 3/µL) developed in up to 2.0% of renal transplant patients, up to 2.8% of cardiac transplant patients and up to 3.6% of hepatic transplant patients receiving mycophenolate mofetil 3 g daily (see WARNINGS: Neutropenia, PRECAUTIONS: Laboratory Tests and DOSAGE AND ADMINISTRATION).
All transplant patients are at increased risk of opportunistic infections. The risk increases with total immunosuppressive load (see WARNINGS: Serious Infections and WARNINGS: New or Reactivated Viral Infections). Table 10 shows the incidence of opportunistic infections that occurred in the renal, cardiac, and hepatic transplant populations in the azathioprine-controlled prevention trials:
Table 10 Viral and Fungal Infections in Controlled Studies in Prevention of Renal, Cardiac or Hepatic Transplant Rejection Renal Studies
Cardiac Study
Hepatic Study
Mycophenolate mofetil
2 g/dayMycophenolate mofetil
3 g/dayAzathioprine
1 to 2 mg/kg/day or 100 to 150 mg/dayMycophenolate mofetil
3 g/dayAzathioprine
1.5 to 3
mg/kg/dayMycophenolate mofetil
3 g/dayAzathioprine
1 to 2
mg/kg/day(n=336)
(n=330)
(n=326)
(n=289)
(n=289)
(n=277)
(n=287)
%
%
%
%
%
%
%
Herpes simplex
16.7
20.0
19.0
20.8
14.5
10.1
5.9
CMV
- Viremia / syndrome
13.4
12.4
13.8
12.1
10.0
14.1
12.2
- Tissue invasive disease
8.3
11.5
6.1
11.4
8.7
5.8
8.0
Herpes zoster
6.0
7.6
5.8
10.7
5.9
4.3
4.9
- Cutaneous disease
6.0
7.3
5.5
10.0
5.5
4.3
4.9
Candida
17.0
17.3
18.1
18.7
17.6
22.4
24.4
- Mucocutaneous
15.5
16.4
15.3
18.0
17.3
18.4
17.4
The following other opportunistic infections occurred with an incidence of less than 4% in mycophenolate mofetil patients in the above azathioprine-controlled studies: Herpes zoster, visceral disease; Candida, urinary tract infection, fungemia/disseminated disease, tissue invasive disease; Cryptococcosis; Aspergillus/Mucor; Pneumocystis carinii.
In the placebo-controlled renal transplant study, the same pattern of opportunistic infection was observed compared to the azathioprine-controlled renal studies, with a notably lower incidence of the following: Herpes simplex and CMV tissue-invasive disease.
In patients receiving mycophenolate mofetil (2 g or 3 g) in controlled studies for prevention of renal, cardiac or hepatic rejection, fatal infection/sepsis occurred in approximately 2% of renal and cardiac patients and in 5% of hepatic patients (see WARNINGS: Serious Infections).
In cardiac transplant patients, the overall incidence of opportunistic infections was approximately 10% higher in patients treated with mycophenolate mofetil than in those receiving azathioprine, but this difference was not associated with excess mortality due to infection/sepsis among patients treated with mycophenolate mofetil.
The following adverse events were reported with 3% to < 20% incidence in renal, cardiac, and hepatic transplant patients treated with mycophenolate mofetil, in combination with cyclosporine and corticosteroids.
Table 11 Adverse Events Reported in 3% to < 20% of Patients Treated With Mycophenolate Mofetil in Combination With Cyclosporine and Corticosteroids Body System
Body as a Whole
abdomen enlarged, abscess, accidental injury, cellulitis, chills occurring with fever, cyst, face edema, flu syndrome, hemorrhage, hernia, lab test abnormal, malaise, neck pain, pelvic pain, peritonitis
Hematologic and Lymphatic
coagulation disorder, ecchymosis, pancytopenia, petechia, polycythemia, prothrombin time increased, thromboplastin time increased
Urogenital
acute kidney failure, albuminuria, dysuria, hydronephrosis, hematuria, impotence, kidney failure, kidney tubular necrosis, nocturia, oliguria, pain, prostatic disorder, pyelonephritis, scrotal edema, urine abnormality, urinary frequency, urinary incontinence, urinary retention, urinary tract disorder
Cardiovascular
angina pectoris, arrhythmia, arterial thrombosis, atrial fibrillation, atrial flutter, bradycardia, cardiovascular disorder, congestive heart failure, extrasystole, heart arrest, heart failure, hypotension, pallor, palpitation, pericardial effusion, peripheral vascular disorder, postural hypotension, pulmonary hypertension, supraventricular tachycardia, supraventricular extrasystoles, syncope, tachycardia, thrombosis, vasodilatation, vasospasm, ventricular extrasystole, ventricular tachycardia, venous pressure increased
Metabolic and Nutritional
abnormal healing, acidosis, alkaline phosphatase increased, alkalosis, bilirubinemia, creatinine increased, dehydration, gamma glutamyl transpeptidase increased, generalized edema, gout, hypercalcemia, hypercholesteremia, hyperlipemia, hyperphosphatemia, hyperuricemia, hypervolemia, hypocalcemia, hypochloremia, hypoglycemia, hyponatremia, hypophosphatemia, hypoproteinemia, hypovolemia, hypoxia, lactic dehydrogenase increased, respiratory acidosis, SGOT increased, SGPT increased, thirst, weight gain, weight loss
Digestive
anorexia, cholangitis, cholestatic jaundice, dysphagia, esophagitis, flatulence, gastritis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gastrointestinal moniliasis, gingivitis, gum hyperplasia, hepatitis, ileus, infection, jaundice, liver damage, liver function tests abnormal, melena, mouth ulceration, nausea and vomiting, oral moniliasis, rectal disorder, stomach ulcer, stomatitis
Respiratory
apnea, asthma, atelectasis, bronchitis, epistaxis, hemoptysis, hiccup, hyperventilation, lung edema, lung disorder, neoplasm, pain, pharyngitis, pleural effusion, pneumonia, pneumothorax, respiratory disorder, respiratory moniliasis, rhinitis, sinusitis, sputum increased, voice alteration
Skin and Appendages
acne, alopecia, fungal dermatitis, hemorrhage, hirsutism, pruritus, rash, skin benign neoplasm, skin carcinoma, skin disorder, skin hypertrophy, skin ulcer, sweating, vesiculobullous rash
Nervous
agitation, anxiety, confusion, convulsion, delirium, depression, dry mouth, emotional lability, hallucinations, hypertonia, hypesthesia, nervousness, neuropathy, paresthesia, psychosis, somnolence, thinking abnormal, vertigo
Endocrine
Cushing's syndrome, diabetes mellitus, hypothyroidism, parathyroid disorder
Musculoskeletal
arthralgia, joint disorder, leg cramps, myalgia, myasthenia, osteoporosis
Special Senses
abnormal vision, amblyopia, cataract (not specified), conjunctivitis, deafness, ear disorder, ear pain, eye hemorrhage, tinnitus, lacrimation disorder
Pediatrics
The type and frequency of adverse events in a clinical study in 100 pediatric patients 3 months to 18 years of age dosed with mycophenolate mofetil oral suspension 600 mg/m 2 bid (up to 1 g bid) were generally similar to those observed in adult patients dosed with mycophenolate mofetil capsules at a dose of 1 g bid with the exception of abdominal pain, fever, infection, pain, sepsis, diarrhea, vomiting, pharyngitis, respiratory tract infection, hypertension, leucopenia, and anemia, which were observed in a higher proportion in pediatric patients.
Mycophenolate Mofetil Intravenous
The adverse event profile of mycophenolate mofetil Intravenous was determined from a single, double-blind, controlled comparative study of the safety of 2 g/day of intravenous and oral mycophenolate mofetil in renal transplant patients in the immediate posttransplant period (administered for the first 5 days). The potential venous irritation of mycophenolate mofetil Intravenous was evaluated by comparing the adverse events attributable to peripheral venous infusion of mycophenolate mofetil Intravenous with those observed in the intravenous placebo group; patients in this group received active medication by the oral route.
Adverse events attributable to peripheral venous infusion were phlebitis and thrombosis, both observed at 4% in patients treated with mycophenolate mofetil Intravenous.
In the active controlled study in hepatic transplant patients, 2 g/day of mycophenolate mofetil Intravenous were administered in the immediate posttransplant period (up to 14 days). The safety profile of intravenous mycophenolate mofetil was similar to that of intravenous azathioprine.
Postmarketing Experience
Congenital Disorders:
Embryofetal Toxicity:
Congenital malformations, including ear, facial, cardiac, and nervous system malformations and an increased incidence of first trimester pregnancy loss have been reported following exposure to mycophenolate mofetil during pregnancy (see PRECAUTIONS: Pregnancy).Digestive
Colitis (sometimes caused by cytomegalovirus), pancreatitis, isolated cases of intestinal villous atrophy.
Hematologic and Lymphatic
Cases of pure red cell aplasia (PRCA) and hypogammaglobulinemia have been reported in patients treated with mycophenolate mofetil in combination with other immunosuppressive agents.
Infections
(See WARNINGS: Serious Infections, New or Reactivated Viral Infections)
- Serious life-threatening infections such as meningitis and infectious endocarditis have been reported occasionally.
- There is evidence of a higher frequency of certain types of serious infections such as tuberculosis and atypical mycobacterial infection.
- Cases of Progressive Multifocal Leukoencephalopathy (PML), sometimes fatal, have been reported in patients treated with mycophenolate mofetil. The reported cases generally had risk factors for PML, including treatment with immunosuppressant therapies and impairment of immune function.
- Polyomavirus associated neuropathy (PVAN), especially due to BK virus infection, has been observed in patients receiving immunosuppressants, including mycophenolate mofetil. This infection is associated with serious outcomes, including deteriorating renal function and renal graft loss.
- Viral reactivation has been reported in patients infected with HBV or HCV.
Respiratory
Interstitial lung disorders, including fatal pulmonary fibrosis, have been reported rarely and should be considered in the differential diagnosis of pulmonary symptoms ranging from dyspnea to respiratory failure in posttransplant patients receiving mycophenolate mofetil.
Call your doctor for medical advice about side effects. You may report side effects to Strides Pharma Inc. at 1-877-244-9825 or go to www.stridesshasun.com.
-
OVERDOSAGE
The experience with overdose of mycophenolate mofetil in humans is very limited. The events received from reports of overdose fall within the known safety profile of the drug. The highest dose administered to renal transplant patients in clinical trials has been 4 g/day. In limited experience with cardiac and hepatic transplant patients in clinical trials, the highest doses used were 4 g/day or 5 g/day. At doses of 4 g/day or 5 g/day, there appears to be a higher rate, compared to the use of 3 g/day or less, of gastrointestinal intolerance (nausea, vomiting, and/or diarrhea), and occasional hematologic abnormalities, principally neutropenia, leading to a need to reduce or discontinue dosing.
In acute oral toxicity studies, no deaths occurred in adult mice at doses up to 4000 mg/kg or in adult monkeys at doses up to 1000 mg/kg; these were the highest doses of mycophenolate mofetil tested in these species. These doses represent 11 times the recommended clinical dose in renal transplant patients and approximately 7 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. In adult rats, deaths occurred after single-oral doses of 500 mg/kg of mycophenolate mofetil. The dose represents approximately 3 times the recommended clinical dose in cardiac transplant patients when corrected for BSA.
MPA and MPAG are usually not removed by hemodialysis. However, at high MPAG plasma concentrations (>100 mcg/mL), small amounts of MPAG are removed. By increasing excretion of the drug, MPA can be removed by bile acid sequestrants, such as cholestyramine (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
-
DOSAGE AND ADMINISTRATION
Renal Transplantation
Adults
A dose of 1 g administered orally or intravenously (over NO LESS THAN 2 HOURS) twice a day (daily dose of 2 g) is recommended for use in renal transplant patients. Although a dose of 1.5 g administered twice daily (daily dose of 3 g) was used in clinical trials and was shown to be safe and effective, no efficacy advantage could be established for renal transplant patients. Patients receiving 2 g/day of mycophenolate mofetil demonstrated an overall better safety profile than did patients receiving 3 g/day of Mycophenolate mofetil.
Pediatrics (3 months to 18 years of age)
The recommended dose of mycophenolate mofetil oral suspension is 600 mg/m 2 administered twice daily (up to a maximum daily dose of 2 g/10 mL oral suspension). Patients with a body surface area of 1.25 m 2 to 1.5 m 2 may be dosed with mycophenolate mofetil capsules at a dose of 750 mg twice daily (1.5 g daily dose). Patients with a body surface area >1.5 m 2 may be dosed with mycophenolate mofetil capsules or tablets at a dose of 1 g twice daily (2 g daily dose).
Mycophenolate Mofetil Capsules and Tablets
The initial oral dose of mycophenolate mofetil should be given as soon as possible following renal, cardiac or hepatic transplantation. Food had no effect on MPA AUC, but has been shown to decrease MPA C max by 40%. Therefore, it is recommended that mycophenolate mofetil be administered on an empty stomach. However, in stable renal transplant patients, mycophenolate mofetil may be administered with food if necessary.
Patients should be instructed to take a missed dose as soon as they remember, except if it is near the next scheduled dose, and then continue to take mycophenolate mofetil at the usual times.
Patients With Hepatic Impairment
No dose adjustments are recommended for renal patients with severe hepatic parenchymal disease. However, it is not known whether dose adjustments are needed for hepatic disease with other etiologies (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
No data are available for cardiac transplant patients with severe hepatic parenchymal disease.
Geriatrics
The recommended oral dose of 1 g bid for renal transplant patients, 1.5 g bid for cardiac transplant patients, and 1.5 g bid administered orally in hepatic transplant patients is appropriate for elderly patients (see PRECAUTIONS: Geriatric Use).
Dosage Adjustments
In renal transplant patients with severe chronic renal impairment (GFR < 25 mL/min/1.73 m 2) outside the immediate posttransplant period, doses of mycophenolate mofetil greater than 1 g administered twice a day should be avoided. These patients should also be carefully observed. No dose adjustments are needed in renal transplant patients experiencing delayed graft function postoperatively (see CLINICAL PHARMACOLOGY: Pharmacokinetics and PRECAUTIONS: Patients with Renal Impairment).
No data are available for cardiac or hepatic transplant patients with severe chronic renal impairment. Mycophenolate mofetil may be used for cardiac or hepatic transplant patients with severe chronic renal impairment if the potential benefits outweigh the potential risks.
If neutropenia develops (ANC < 1.3 x 10 3/µL), dosing with mycophenolate mofetil should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately (see WARNINGS: Neutropenia, ADVERSE REACTIONS, and PRECAUTIONS: Laboratory Tests).
- HANDLING AND DISPOSAL
-
HOW SUPPLIED
Mycophenolate Mofetil Capsules, USP 250 mg
White to off-white blend of mycophenolate mofetil filled in size '1' hard gelatin capsule with Ivory Cap and Ivory Body, printed 'SAL' on cap and '726' on body in black. Supplied in the following presentations:
Unit dose packages of 100 (10 x 10) NDC: 60687-427-01Storage
Store at 20° to 25°C (68° to 77°F). [See USP controlled room temperature].Mycophenolate Mofetil Tablets, USP 500 mg
Pinkish brown colored, capsule shaped, film coated tablet with "SAL" engraved on one side and engraved "725" on other side. Supplied in the following presentations:
Unit dose packages of 100 (10 x 10) NDC: 60687-438-01Storage and Dispensing Information:
Store at 20º to 25°C (68º to 77°F) [see USP controlled room temperature].FOR YOUR PROTECTION: Do not use if blister is torn or broken.
-
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section) contain drug product from Strides Pharma Inc. as follows:
(250 mg / 100 UD) NDC: 60687-427-01 packaged from NDC: 64380-726
(250 mg / 100 UD) NDC: 60687-438-01 packaged from NDC: 64380-725Distributed by:
American Health Packaging
Columbus, OH 432178442701/1218
-
MEDICATION GUIDE
8442701/1218
Mycophenolate mofetil capsules, USP
Mycophenolate mofetil tablets, USPRead the Medication Guide that comes with mycophenolate mofetil before you start taking it and each time you refill your prescription. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about mycophenolate mofetil?
Mycophenolate mofetil can cause serious side effects:-
Increased risk of loss of a pregnancy (miscarriage) and higher risk of birth defects. Females who take mycophenolate mofetil during pregnancy have a higher risk of
miscarriage during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects.
If you are a female who can become pregnant:- your doctor must talk with you about acceptable birth control methods (contraceptive counseling) to use while taking mycophenolate mofetil.
- you should have one pregnancy test immediately before starting mycophenolate mofetil tablets and another pregnancy test 8 to 10 days later. Pregnancy tests should be repeated during routine follow-up visits with your doctor. Talk to your doctor about the results of all of your pregnancy tests.
- you must use acceptable birth control, during your entire mycophenolate mofetil tablets therapy and for 6 weeks after stopping mycophenolate mofetil tablets, unless at any time you choose to avoid sexual intercourse (abstinence) with a man completely.
Mycophenolate mofetil decreases blood levels of the hormones in birth control pills that you take by mouth. Birth control pills may not work as well while you take mycophenolate mofetil, and you could become pregnant. If you take birth control pills while using mycophenolate mofetil you must also use another form of birth control. Talk to your doctor about other birth control methods that you can use while taking mycophenolate mofetil.
If you plan to become pregnant, talk with your doctor. Your doctor will decide if other medicines to prevent rejection may be right for you.
If you become pregnant while taking mycophenolate mofetil, do not stop taking Mycophenolate mofetil. Call your doctor right away. In certain situations, you and your doctor may decide that taking mycophenolate mofetil is more important to your health than the possible risks to your unborn baby.
- You and your doctor should report your pregnancy to
Mycophenolate Pregnancy Registry (1-800-617-8191)
The purpose of this registry is to gather information about the health of you and your baby.
- Increased risk of getting serious infections. Mycophenolate mofetil weakens the body's immune system and affects your ability to fight infections. Serious infections can happen with mycophenolate mofetil and can lead to death. Types of infections can include:
- Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with mycophenolate mofetil include:
- Shingles, other herpes infections, and cytomegalovirus (CMV). CMV can cause serious tissue and blood infections.
- BK virus. BK virus can affect how your kidney works and cause your transplanted kidney to fail.
- Hepatitis B and C viruses. Hepatitis viruses can affect how your liver works. Talk to your doctor about how hepatitis viruses may affect you.
- A brain infection called Progressive Multifocal Leukoencephalopathy (PML). In some patients, mycophenolate mofetil may cause an infection of the brain that may cause death. You are at risk for this brain infection because you have a weakened immune system. You should tell your doctor right away if you have any of the following symptoms:
- Weakness on one side of the body
- You do not care about things that you usually care about (apathy)
- You are confused or have problems thinking
- You cannot control your muscles
- Fungal infections. Yeasts and other types of fungal infections can happen with mycophenolate mofetil and can cause serious tissue and blood infections (see “What are the possible side effects of Mycophenolate mofetil?”).
Call your doctor right away if you have any of the following signs and symptoms of infection:
-
- Temperature of 100.5°F or greater
- Cold symptoms, such as a runny nose or sore throat
- Flu symptoms, such as an upset stomach, stomach pain, vomiting or diarrhea
- Earache or headache
- Pain during urination
- White patches in the mouth or throat
- Unexpected bruising or bleeding
- Cuts, scrapes or incisions that are red, warm and oozing pus
-
Increased risk of getting certain cancers. People who take mycophenolate mofetil have a higher risk of getting lymphoma, and other cancers, especially skin cancer. Tell your doctor if you have:
- unexplained fever, prolonged tiredness, weight loss or lymph node swelling.
- a brown or black skin lesion with uneven borders, or one part of the lesion does not look like the other
- a change in the size and color of a mole
- a new skin lesion or bump
- any other changes to your health
See the section “What are the possible side effects of mycophenolate mofetil?” for information about other serious side effects.
What is Mycophenolate mofetil?
Mycophenolate mofetil is a prescription medicine to prevent rejection (antirejection medicine) in people who have received a kidney, heart or liver transplant. Rejection is when the body's immune system perceives the new organ as a “foreign” threat and attacks it.Mycophenolate mofetil is used with other medicines called cyclosporines (Sandimmune ®, Gengraf ®, Neoral ®) and corticosteroids.
Mycophenolate mofetil has been used safely and works in children who received a kidney transplant as it does in adults. It is not known if mycophenolate mofetil is safe and works in children who receive a heart or liver transplant.
Who should not take Mycophenolate mofetil?
Do not take mycophenolate mofetil if you are allergic to mycophenolate mofetil or any of the ingredients in mycophenolate mofetil capsules, USP and mycophenolate mofetil tablets, USP. See the end of this Medication Guide for a complete list of ingredients in mycophenolate mofetil capsules USP and mycophenolate mofetil tablets USP.What should I tell my doctor before taking mycophenolate mofetil?
Tell your doctor about all of your medical conditions, if you:- have any digestive problems, such as ulcers.
- have Phenylketonuria (PKU).
-
have Lesch-Nyhan or Kelley-Seegmiller syndrome or another rare inherited deficiency hypoxanthine-guanine phosphoribosyl-transfe
rase (HGPRT). You should not take mycophenolate mofetil if you have one of these disorders.
- plan to receive any vaccines. People taking Mycophenolate mofetil should not take live vaccines. Some vaccines may not work as well during treatment with mycophenolate mofetil.
- are pregnant or are planning to become pregnant. See “What is the most important information I should know about mycophenolate mofetil?”
- are breastfeeding or plan to breastfeed. It is not known if mycophenolate mofetil passes into breast milk. You and your doctor will decide if you will take mycophenolate mofetil or breastfeed.
Tell your healthcare provider about all of the medicines you are taking including prescription and nonprescription medicines, vitamins and herbal supplements. Some medicines may affect the way mycophenolate mofetil works, and mycophenolate mofetil may affect how some medicines work. Especially tell your doctor if you take:
- birth control pills (oral contraceptives). See “What is the most important information I should know about mycophenolate mofetil?”
- sevelamer (Renagel ®, Renvela™). These products should be taken 2 hours after taking mycophenolate mofetil.
- acyclovir (Zovirax ®), valacyclovir (Valtrex ®), ganciclovir (CYTOVENE ® –IV, Vitrasert ®), valganciclovir (VALCYTE ®).
- rifampin (Rifater ®, Rifamate ®, Rimactane ®, Rifadin ®).
- antacids that contain magnesium and aluminum (Mycophenolate mofetil and the antacid should not be taken at the same time).
- proton pump inhibitors (PPIs) (Prevacid ®, Protonix ®).
- sulfamethoxazole/trimethoprim (BACTRIM™, BACTRIM DS™).
- norfloxacin (Noroxin ®) and metronidazole (Flagyl ®, Flagyl ® ER, Flagyl ® IV, Metro IV, Helidac ®, Pylera™).
- ciprofloxacin (Cipro ®, Cipro ® XR, Ciloxan ®, Proquin ® XR) and amoxicillin plus clavulanic acid (Augmentin ®, Augmentin XR™).
- azathioprine (Azasan ®, Imuran ®).
- cholestyramine (Questran Light ®, Questran ®, Locholest Light, Locholest, Prevalite ®).
Know the medicines you take. Keep a list of them to show to your doctor or nurse and pharmacist when you get a new medicine. Do not take any new medicine without talking with your doctor.
How should I take mycophenolate mofetil?
- Take mycophenolate mofetil tablets exactly as prescribed.
- Do not stop taking mycophenolate mofetil or change the dose unless your doctor tells you to.
- If you miss a dose of mycophenolate mofetil, or are not sure when you took your last dose, take the regular amount of mycophenolate mofetil prescribed as soon as you remember. If it is time for your next dose, skip the missed dose and take your next dose at your normal scheduled time. Do not take 2 doses at the same time. Call your doctor if you are not sure what to do.
- Take mycophenolate mofetil capsules, USP and mycophenolate mofetil tablets, USP on an empty stomach, either 1 hour before or 2 hours after a meal, unless your healthcare provider tells you otherwise. With the approval of your healthcare provider, in stable kidney transplant patients, mycophenolate Mofetil can be taken with food if necessary.
- Most people take mycophenolate Mofetil by mouth either as ivory capsules or as pinkish brown tablets. Some people may get mycophenolate mofetil soon after their transplant surgery as an infusion into a vein.
- Do not crush mycophenolate mofetil tablets, USP. Do not open or crush mycophenolate mofetil capsules, USP.
- If you take too much mycophenolate mofetil, call your doctor or the poison control center right away.
What should I avoid while taking mycophenolate mofetil?
- Avoid pregnancy. See “What are the most important information I should know about mycophenolate mofetil?”
- Limit the amount of time you spend in sunlight. Avoid using tanning beds or sunlamps. People who take mycophenolate mofetil have a higher risk of getting skin cancer. (See “What is the most important information I should know about Mycophenolate Mofetil?”) Wear protective clothing when you are in the sun and use a sunscreen with a high protection factor (SPF 30 and above). This is especially important if your skin is very fair or if you have a family history of skin cancer.
What are the possible side effects of mycophenolate mofetil?Mycophenolate mofetil can cause serious side effects:
- See “What is the most important information I should know about mycophenolate mofetil?”
-
Low blood cell counts. People taking high doses of mycophenolate mofetil each day may have a decrease in blood counts, including.
- White blood cells, especially neutrophils. Neutrophils fight against bacterial infections. You have a higher chance of getting an infection when your white blood cell count is low. This is most common from 3 months to 6 months after your transplant.
- red blood cells. Red blood cells carry oxygen to your body tissues. You have a higher chance of getting severe anemia when your red blood cell count is low.
- platelets. Platelets help with blood clotting.
Your doctor will do blood tests before you start taking mycophenolate mofetil and during treatment with mycophenolate mofetil to check your blood cell counts.
Tell your doctor right away if you have any signs of infection (see “What is the most important information I should know about mycophenolate mofetil?”), or any unexpected bruising or bleeding. Also, tell your doctor if you have unusual tiredness, lack of energy, dizziness or fainting.
- Stomach problems. Stomach and intestinal bleeding can happen in people who take high doses of mycophenolate mofetil. Bleeding can be severe and you may have to be hospitalized for treatment.
Common side effects include:
-
- diarrhea. Call your doctor right away if you have diarrhea. Do not stop taking Mycophenolate mofetil without first talking with your doctor.
- vomiting.
- pain.
- stomach area pain.
- swelling of the lower legs, ankles and feet.
- high blood pressure.
Side effects that happen more often in children than in adults taking mycophenolate mofetil include:
- stomach area pain
- sore throat
- fever
- colds (respiratory tract infections)
- infection
- high blood pressure
- pain
- low white blood cell count
- blood infection (sepsis)
- Low red blood cell count
- diarrhea
- vomiting
These are not all of the possible side effects to mycophenolate mofetil. Tell your doctor about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or Strides Pharma Inc. at 1-877-244-9825 or go to www.stridesshasun.com.
How should I store mycophenolate mofetil?
- Store mycophenolate mofetil tablets USP and mycophenolate mofetil capsules USP at at 20º to 25°C (68º to 77°F). [See USP controlled room temperature].
- Keep mycophenolate mofetil and all medicines out of the reach of children.
General Information about mycophenolate mofetil
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mycophenolate mofetil for a condition for which it was not prescribed. Do not give mycophenolate mofetil to other people, even if they have the same symptoms that you have. It may harm them.This Medication Guide summarizes the most important information about mycophenolate mofetil. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about mycophenolate mofetil that is written for healthcare professionals. For more information call Sandoz Inc. at 1-800-438-1985.
What are the ingredients in mycophenolate mofetil?
Active Ingredient: Mycophenolate MofetilMycophenolate mofetil capsules, USP 250 mg: Croscarmellose sodium, magnesium stearate, povidone (K-30) and microcrystalline cellulose. The capsule shells contain yellow iron oxide, FD & C red # 3, gelatin, sodium lauryl sulfate, and titanium dioxide.
Mycophenolate mofetil tablets, USP 500 mg: Microcrystalline cellulose, croscarmellose sodium, povidone [K-30], magnesium stearate (Vegetable), opadry brown, opacode black. The opadry brown contains FD & C blue #1 aluminum lake, FD & C red #40 aluminum lake, hypromellose, iron oxide red, polyethylene glycol and titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
American Health Packaging
Columbus, OH 432178442701/1218
-
Increased risk of loss of a pregnancy (miscarriage) and higher risk of birth defects. Females who take mycophenolate mofetil during pregnancy have a higher risk of
miscarriage during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects.
-
Package/Label Display Panel – Carton – 250 mg

NDC 60687- 427-01
Mycophenolate
Mofetil Capsules, USP250 mg
100 Capsules (10 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.Each Capsule Contains:
Mycophenolate mofetil, USP...................................... 250 mgUsual Dosage: See package insert for full prescribing
information.CAUTION: Special handling and Disposal instructions see
insert.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 64380-726, Strides Pharma Inc.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217742701
0442701/1218 - Package/Label Display Panel – Blister – 250 mg
-
Package/Label Display Panel – Carton – 500 mg

NDC 60687- 438-01
Mycophenolate
Mofetil Tablets, USP500 mg
100 Tablets (10 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.Each Film-Coated Tablet Contains:
Mycophenolate mofetil, USP......................................500 mgUsual Dosage: See package insert for full prescribing
information.CAUTION: Special handling and Disposal instructions see
insert.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 64380-725, Strides Pharma Inc.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217743801
0443801/1218 - Package/Label Display Panel – Blister – 500 mg
-
INGREDIENTS AND APPEARANCE
MYCOPHENOLATE MOFETIL
mycophenolate mofetil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60687-427(NDC:64380-726) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MYCOPHENOLATE MOFETIL (UNII: 9242ECW6R0) (MYCOPHENOLIC ACID - UNII:HU9DX48N0T) MYCOPHENOLATE MOFETIL 250 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code SAL726 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60687-427-01 100 in 1 BOX, UNIT-DOSE 05/15/2019 1 NDC: 60687-427-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090055 05/15/2019 MYCOPHENOLATE MOFETIL
mycophenolate mofetil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60687-438(NDC:64380-725) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MYCOPHENOLATE MOFETIL (UNII: 9242ECW6R0) (MYCOPHENOLIC ACID - UNII:HU9DX48N0T) MYCOPHENOLATE MOFETIL 500 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color brown Score no score Shape CAPSULE Size 18mm Flavor Imprint Code SAL725 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60687-438-01 100 in 1 BOX, UNIT-DOSE 05/15/2019 1 NDC: 60687-438-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090456 05/15/2019 Labeler - American Health Packaging (929561009) Establishment Name Address ID/FEI Business Operations American Health Packaging 929561009 repack(60687-427, 60687-438)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

