Mark 3 by Cargus International, Inc. / Dharma Research, Inc. MARK 3- benzocaine gel
Mark 3 by
Drug Labeling and Warnings
Mark 3 by is a Otc medication manufactured, distributed, or labeled by Cargus International, Inc., Dharma Research, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
-
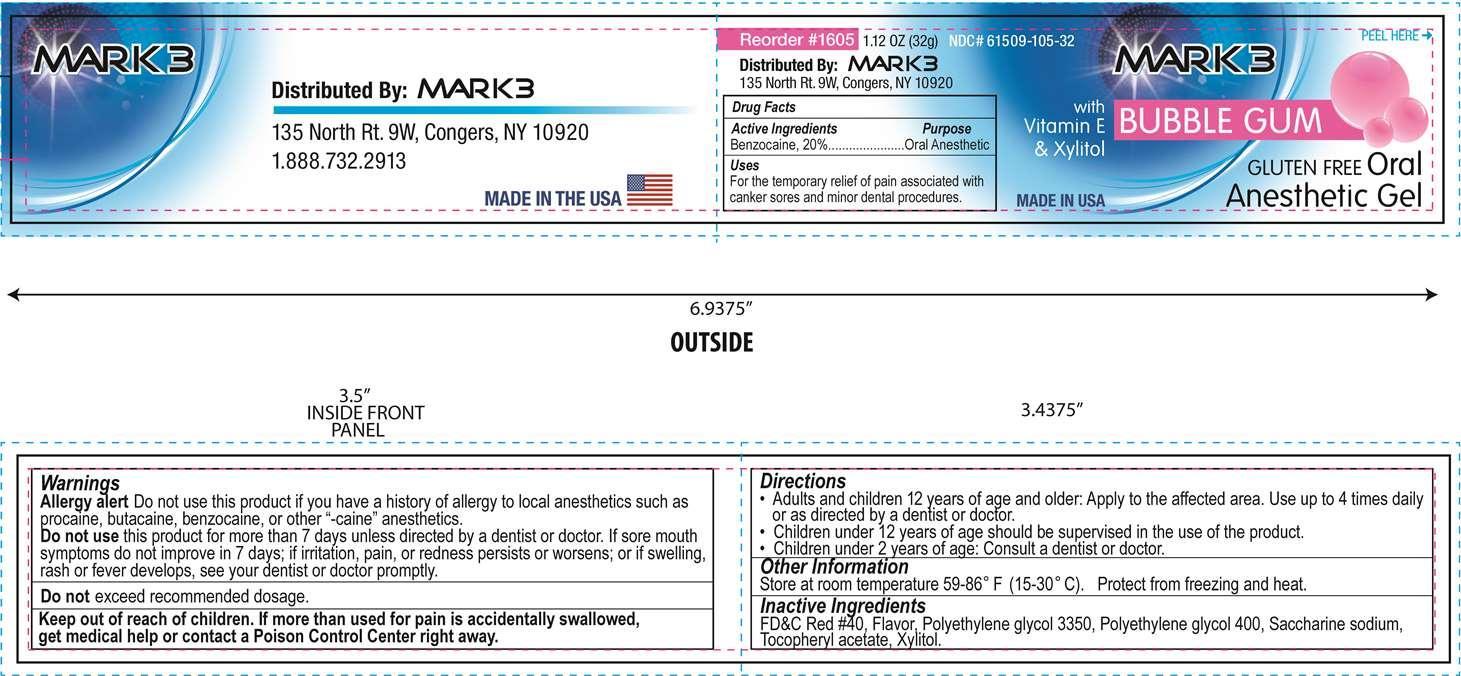
Warnings
Allergy alert Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "-caine" anesthetics.
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- Distributed by
-
PRINCIPAL DISPLAY PANEL
Mark 3
Oral Anesthetic Gel
Bubble Gum
Gluten Free
with Vitamin E and Xylitol
NDC: 61509-105-32
Net Wt. 1.12 oz. (32 g)
Distributed by Mark 3, 135 North Rt. 9W, Congers, NY 10920
Made in USA
-
INGREDIENTS AND APPEARANCE
MARK 3
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61509-105 Route of Administration ORAL, DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6.4 g in 32 g Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SACCHARIN SODIUM (UNII: SB8ZUX40TY) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61509-105-32 32 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 03/03/2014 Labeler - Cargus International, Inc. (096191093) Establishment Name Address ID/FEI Business Operations Dharma Research, Inc. 078444642 manufacture(61509-105)
Trademark Results [Mark 3]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MARK 3 88866323 not registered Live/Pending |
Leupold & Stevens, Inc. 2020-04-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.