SINUS CONGESTION- phenylephrine hcl tablet, film coated
Sinus Congestion by
Drug Labeling and Warnings
Sinus Congestion by is a Otc medication manufactured, distributed, or labeled by L.N.K. International, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- diabetes
- thyroid disease
- high blood pressure
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
QUALITY
+PLUSNDC: 50844-493-08
*Compare to active ingredient in Sudafed PE® Sinus Congestion
MAXIMUM STRENGTH
SINUS CONGESTION
Phenylephrine HCl 10 mg NASAL DECONGESTANTSINUS PRESSURE
SINUS CONGESTION24 Tablets
NON-DROWSY
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Sudafed PE® Sinus Congestion.
50844 ORG082045308Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788
USA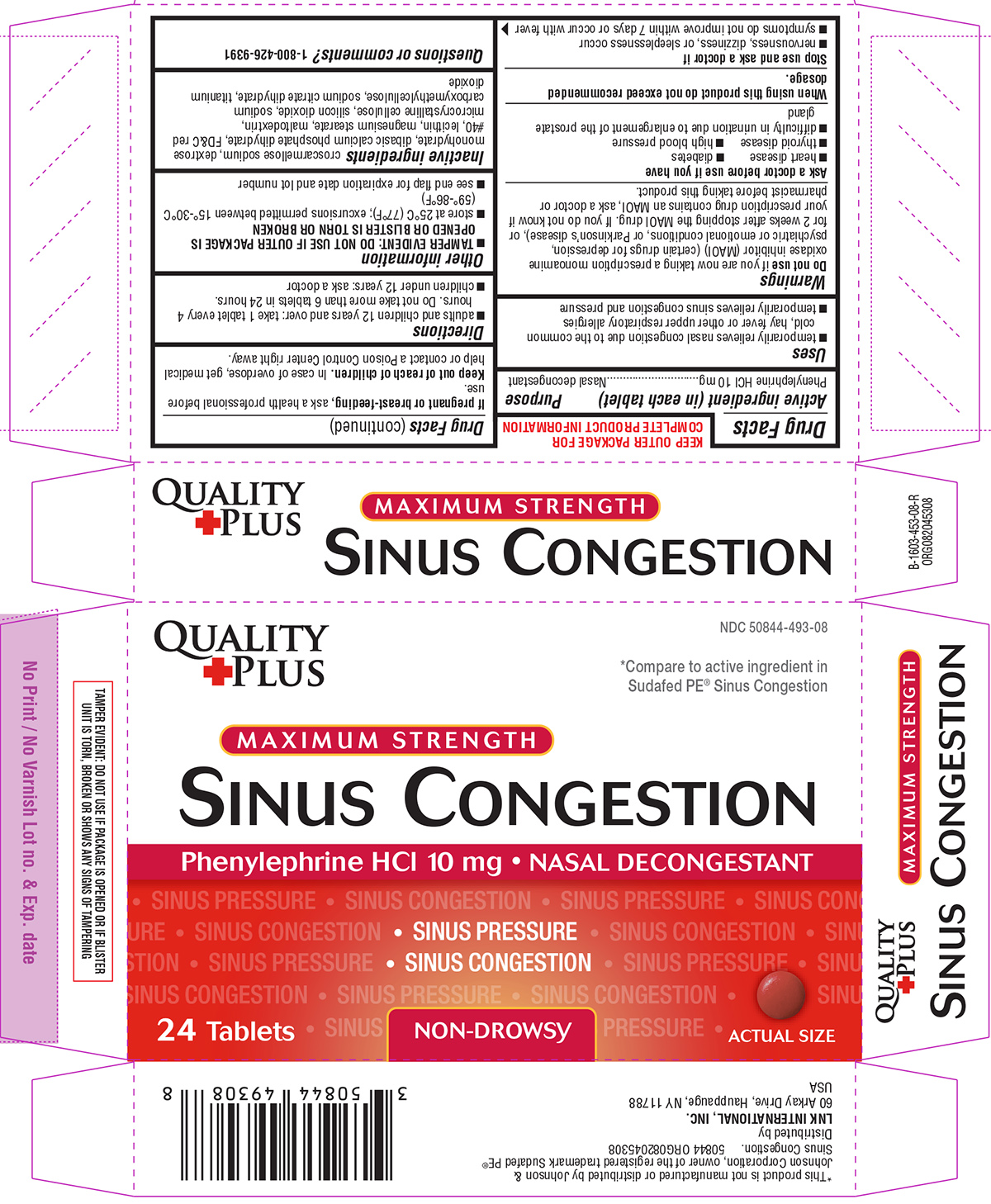
Quality Plus 44-453
-
INGREDIENTS AND APPEARANCE
SINUS CONGESTION
phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50844-493 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 7mm Flavor Imprint Code 44;453 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50844-493-08 1 in 1 CARTON 07/14/2021 1 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 50844-493-07 2 in 1 CARTON 05/17/2023 2 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/14/2021 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(50844-493) , pack(50844-493) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(50844-493) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(50844-493) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(50844-493)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.