Allergy Relief DIPHENHYDRAMINE HCI, 25 mg Antihistamine
Allergy Relief DIPHENHYDRAMINE HCI, 25 mg Antihistamine by
Drug Labeling and Warnings
Allergy Relief DIPHENHYDRAMINE HCI, 25 mg Antihistamine by is a Otc medication manufactured, distributed, or labeled by MEIJER DISTRIBUTION, INC., USpharma Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALLERGY RELIEF DIPHENHYDRAMINE HCI, 25 MG ANTIHISTAMINE- diphenhydramine hcl bar, chewable
MEIJER DISTRIBUTION, INC.
----------
Allergy Relief DIPHENHYDRAMINE HCI, 25 mg Antihistamine
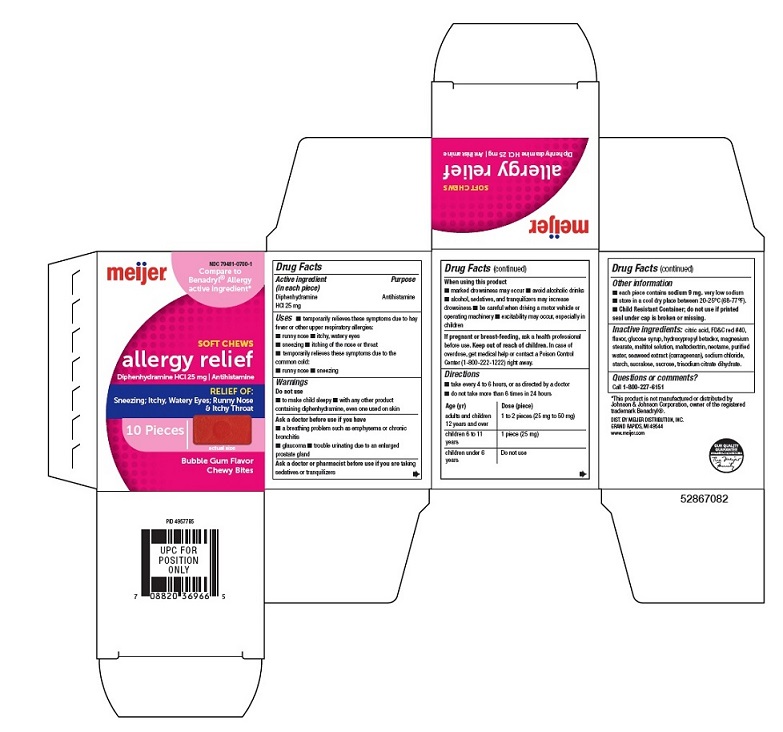
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- To make child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hour
Age (yr)
Dose (Piece)
Adults and children 12 years and over
1 to 2 Pieces (25 mg to 50 mg)
Children 6 to 11 years
1 Piece (25 mg)
Children under 6 years
Do not use
Other information
- each piece: contains sodium 9 mg.
very low sodium
- store in a cool dry place between 20-25°C (68-77°F).
- Child Resistant Container; do not use if printed seal under cap is broken or missing.
Inactive ingredients:
citric acid, FD&C red #40, flavor, glucose syrup, hydroxypropyl betadex, magnesium stearate, maltitol solution, maltodextrin, neotame, purified water, seaweed extract (carrageenan), sodium chloride, starch, sucralose, sucrose, trisodium citrate dihydrate.
Principal display Panel-25 mg carton label
NDC 79481-0700-1
Compare to the active ingredient in Benadryl ® Allergy*
SOFT CHEWS
allergy relief Diphenhydramine HCl, 25 mg | Antihistamine
RELIEF OF:
Sneezing; Itchy, Watery Eyes; Runny Nose & Itchy Throat
10 Pieces actual size
Bubble Gum Flavor Chewy Bites
| ALLERGY RELIEF DIPHENHYDRAMINE HCI, 25 MG ANTIHISTAMINE
diphenhydramine hcl bar, chewable |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - MEIJER DISTRIBUTION, INC. (006959555) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| USpharma Ltd | 080664601 | manufacture(79481-0700) , pack(79481-0700) | |