DOCUSATE SODIUM capsule, liquid filled
DOCUSATE SODIUM by
Drug Labeling and Warnings
DOCUSATE SODIUM by is a Otc medication manufactured, distributed, or labeled by SPIRIT PHARMACEUTICALS,LLC, SOFTGEL HEALTHCARE PVT LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- Directions
- Inactive ingredients
-
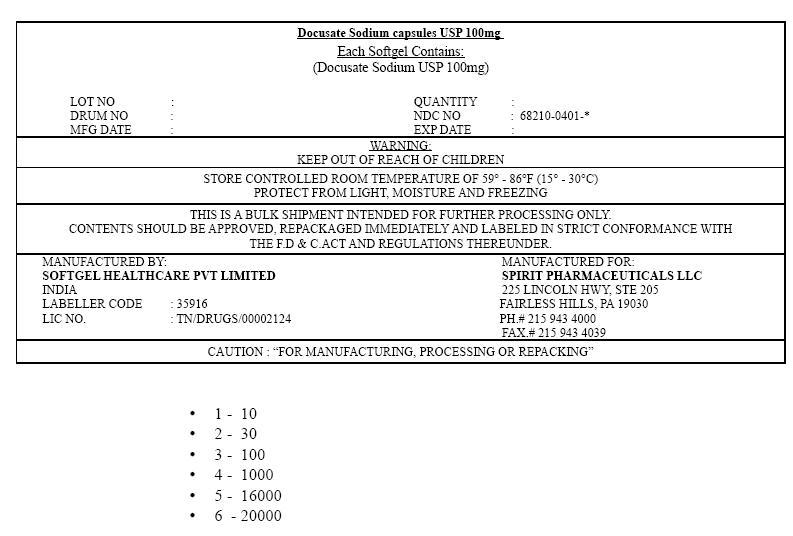
PRINCIPAL DISPLAY PANEL
Docusate Sodium capsules USP 100mg
Each Softgel Contains:
(Docusate Sodium USP 100mg)LOT NO : QUANTITY : DRUM NO : NDC NO : 68210-0401-* MFG DATE : EXP DATE : WARNING:
KEEP OUT OF REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPERATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT CONFORMANCE WITH
THE F.D & C.ACT AND REGULATIONS THEREUNDER.MANUFACTURED BY:
SOFTGEL HEALTHCARE PVT LIMITED
INDIA
LABELLER CODE : 35916
LIC NO. : TN/DRUGS/00002124MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS, PA 19030
PH.# 215 943 4000
FAX.# 215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
- 1 - 10
- 2 - 30
- 3 - 100
- 4 - 1000
- 5 - 16000
- 6 - 20000
-
INGREDIENTS AND APPEARANCE
DOCUSATE SODIUM
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68210-0401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) GLYCERIN (UNII: PDC6A3C0OX) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) D&C RED No. 33 (UNII: 9DBA0SBB0L) FD&C RED No. 40 (UNII: WZB9127XOA) FD&C Yellow No. 6 (UNII: H77VEI93A8) Product Characteristics Color RED Score no score Shape OVAL Size 10mm Flavor Imprint Code DO4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68210-0401-1 10 in 1 BOX 2 NDC: 68210-0401-2 30 in 1 BOX 3 NDC: 68210-0401-3 100 in 1 BOX 4 NDC: 68210-0401-4 1000 in 1 BOX 5 NDC: 68210-0401-5 16000 in 1 BOX 6 NDC: 68210-0401-6 20000 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 03/01/2010 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PVT LTD 675584180 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.