MESALAMINE capsule, extended release
MESALAMINE by
Drug Labeling and Warnings
MESALAMINE by is a Prescription medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Inc., Alembic Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESALAMINE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for MESALAMINE EXTENDED-RELEASE CAPSULES.
MESALAMINE extended-release capsules, for oral use
Initial U.S. Approval: 1987
RECENT MAJOR CHANGES
Warnings and Precautions
Renal Impairment (5.1) 11/2022
INDICATIONS AND USAGE
Mesalamine extended-release capsule is an aminosalicylate indicated for the maintenance of remission of ulcerative colitis in adults. (1) (1)
DOSAGE AND ADMINISTRATION
Dosage
The recommended dosage is 1.5 g (four 0.375 g capsules) once daily in the morning. (2) (2)
Administration Instructions
Evaluate renal function before initiating therapy with mesalamine extended-release capsules. (2)
Swallow the capsules whole. Do not cut, break, crush or chew the capsules. (2)
Avoid co-administration with antacids. (2, 7.1)
Drink an adequate amount of fluids. (2, 5.7)
Take mesalamine extended-release capsules without regard to meals. (2) (2)DOSAGE FORMS AND STRENGTHS
Extended-release capsules: 0.375 g (3) (3)
CONTRAINDICATIONS
Known or suspected hypersensitivity to salicylates, aminosalicylates, or any component of mesalamine extended-release capsule. (4, 5.3) (4)
WARNINGS AND PRECAUTIONS
Renal Impairment: Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits in patients with known renal impairment or taking nephrotoxic drugs; monitor renal function. Discontinue if renal function deteriorates.(5.1, 7.2, 8.6)
Mesalamine-Induced Acute Intolerance Syndrome:Symptoms may be difficult to distinguish from an exacerbation of ulcerative colitis; monitor for worsening symptoms; discontinue treatment if acute intolerance syndrome is suspected. (5.2)
Hypersensitivity Reactions, including Myocarditis and Pericarditis: Evaluate patients immediately and discontinue if a hypersensitivity reaction is suspected. (5.3)
Hepatic Failure: Evaluate the risks and benefits in patients with known liver impairment. (5.4)
Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
Photosensitivity: Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors. (5.6)
Nephrolithiasis: Mesalamine-containing stones are undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment. (5.7)
Interference with Laboratory Tests:Use of mesalamine may lead to spuriously elevated test results when measuring urinary Normetanephrine by liquid chromatography with electrochemical detection. (5.9) (5)ADVERSE REACTIONS
DRUG INTERACTIONS
Nephrotoxic Agents including NSAIDs: Increased risk of nephrotoxicity; monitor for changes in renal function and mesalamine-related adverse reactions. (7.2)
Azathioprine or 6-Mercaptopurine: Increased risk of blood disorders; monitor complete blood cell counts and platelet counts. (7.3) (7)
USE IN SPECIFIC POPULATIONS
Geriatric Patients: Increased risk of blood dyscrasias; monitor blood cell counts and platelet counts. (8.5) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Mesalamine-Induced Acute Intolerance Syndrome
5.3 Hypersensitivity Reactions
5.4 Hepatic Failure
5.5 Severe Cutaneous Adverse Reactions
5.6 Photosensitivity
5.7 Nephrolithiasis
5.9 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antacids
7.2 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
7.3 Azathioprine or 6-Mercaptopurine
7.4 Interference with Urinary Normetanephrine Measurements
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Dosage
The recommended dosage in adults is 1.5 g (four 0.375 g capsules) orally once daily in the morning.
Administration Instructions
Evaluate renal function before initiating therapy with mesalamine extended-release capsules [see Warnings and Precautions (5.1)].
Swallow mesalamine extended-release capsules whole. Do not cut, break, crush or chew the capsules.
Avoid co-administration of mesalamine extended-release capsules with antacids [see Drug Interactions (7.1)].
Drink an adequate amount of fluids [see Warnings and Precautions (5.7)].
Take mesalamine extended-release capsules without regard to meals [see Clinical Pharmacology (12.3)]. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure, has been reported in patients given products such as mesalamine that contain mesalamine or are converted to mesalamine. In animal studies, the kidney was the principal organ of mesalamine toxicity [see Adverse Reactions (6.2), Nonclinical Toxicology (13.2)].
Evaluate renal function prior to initiation of mesalamine therapy and periodically while on therapy. Evaluate the risks and benefits of using mesalamine in patients with known renal impairment or a history of renal disease or taking concomitant nephrotoxic drugs. Discontinue mesalamine if renal function deteriorates while on therapy [see Drug Interactions (7.2), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with mesalamine.
5.3 Hypersensitivity Reactions
Some patients have experienced a hypersensitivity reaction to sulfasalazine. Some patients may have a similar reaction to mesalamine or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue mesalamine if an alternative etiology for the signs and symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Evaluate the risks and benefits of using mesalamine in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine [see Adverse Reactions (6.2)]. Discontinue mesalamine at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.7 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with mesalamine.
5.9 Interference with Laboratory Tests
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
Renal Impairment [see Warnings and Precautions (5.1)]
Mesalamine-Induced Acute Intolerance Syndrome [see Warnings and Precautions (5.2)]
Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
Hepatic Failure [see Warnings and Precautions (5.4)]
Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)]
Photosensitivity [see Warnings and Precautions (5.6)]
Nephrolithiasis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to mesalamine in 557 patients, including 354 exposed for at least 6 months and 250 exposed for greater than one year. Mesalamine was studied in two placebo-controlled trials (n=367 treated with mesalamine) and in one open-label, long-term study (n=190 additional patients). The population consisted of patients with ulcerative colitis; the mean age was 47 years, 54% were female, and 93% were white. Patients received doses of mesalamine 1.5 g administered orally once per day for six months in the placebo-controlled trials and for up to 24 months in the open-label study.
In the two placebo-controlled trials, the most common reactions reported in at least 3% of mesalamine-treated patients and at a greater rate than placebo are shown in Table 1 below.
Table 1: Common Adverse Reactions* in Clinical Trials of Adults with Ulcerative Colitis
Mesalamine
1.5 g once daily
N=367
Placebo
N=185
Headache
11%
8%
Diarrhea
8%
7%
Upper Abdominal Pain
5%
3%
Nausea
4%
3%
Nasopharyngitis
4%
3%
* Reported in at least 3% of mesalamine-treated patients and at a greater rate than with placebo
The following adverse reactions, presented by body system, were reported at a frequency less than 3% in patients treated with mesalamine for up to 24 months in controlled and open-label trials.
Ear and Labyrinth Disorders: tinnitus, vertigo
Dermatological Disorder: alopecia
Gastrointestinal: lower abdominal pain, rectal hemorrhage
Laboratory Abnormalities: increased triglycerides, decreased hematocrit and hemoglobin
General Disorders and Administration Site Disorders: fatigue
Hepatic: hepatitis cholestatic, transaminases increased
Renal Disorders: creatinine clearance decreased, hematuria
Musculoskeletal: pain, arthralgia
Respiratory: dyspnea
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of mesalamine or other mesalamine-containing products. Because many of these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: lupus-like syndrome, drug fever
Cardiovascular: pericarditis, pericardial effusion, myocarditis [see Warnings and Precautions (5.3)]
Gastrointestinal: pancreatitis, cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer
Hepatic: jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, Kawasaki-like syndrome including changes in liver enzymes
Hematologic: agranulocytosis, aplastic anemia
Nervous System: intracranial hypertension
Neurological/Psychiatric: peripheral neuropathy, Guillain-Barré syndrome, transverse myelitis
Renal and Urinary: nephrogenic diabetes insipidus, interstitial nephritis, renal failure, minimal change disease, nephrolithiasis [see Warnings and Precautions (5.1, 5.7)]
Urine discoloration occurring ex-vivo caused by contact of mesalamine, including inactive metabolite, with surfaces or water treated with hypochlorite-containing bleach.
Respiratory/Pulmonary: eosinophilic pneumonia, interstitial pneumonitis, pleurisy/pleuritis
Skin: psoriasis, pyoderma gangrenosum, erythema nodosum, SJS/TEN, DRESS, and AGEP [see Warnings and Precautions (5.5)]
Renal/Urogenital: reversible oligospermia
-
7 DRUG INTERACTIONS
7.1 Antacids
Because the dissolution of the coating of the granules in mesalamine extended-release capsules depends on pH, avoid co-administration of
mesalamine extended-release capsules with antacids [see Dosage and Administration (2)].7.2 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.3 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of mesalamine and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.4 Interference with Urinary Normetanephrine Measurements
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection [see Warnings and Precautions (5.9)]. Consider an alternative, selective assay for normetanephrine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from meta-analyses, cohort studies and case series on the use of mesalamine during pregnancy have not reliably informed an association with mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data).
In animal reproduction studies, there were no adverse developmental outcomes with administration of oral mesalamine during organogenesis to pregnant rats and rabbits at doses 1.7 and 5.4 times, respectively, the maximum recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Data
Human Data
Published data from meta-analyses, cohort studies and case series on the use of mesalamine during early pregnancy (first trimester) and throughout pregnancy have not reliably informed an association of mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is no clear evidence that mesalamine exposure in early pregnancy is associated with an increased risk in major congenital malformations, including cardiac malformations. Published epidemiologic studies have important methodological limitations which hinder interpretation of the data, including inability to control for confounders, such as underlying maternal disease, and maternal use of concomitant medications, and missing information on the dose and duration of use for mesalamine products.
Animal Data
Reproduction studies with mesalamine during organogenesis have been performed in rats at oral doses up to 320 mg/kg/day (about 1.7 times the recommended human dose based on a body surface area comparison) and rabbits at doses up to 495 mg/kg/day (about 5.4 times the recommended human dose based on a body surface area comparison) and have revealed no evidence of harm to the fetus due to mesalamine.
8.2 Lactation
Risk Summary
Data from published literature report the presence of mesalamine and its metabolite, N-acetyl 5-aminosalicylic acid in human milk in small amounts with relative infant doses (RID) of 2% or less (see Data). There are case reports of diarrhea in breastfed infants exposed to mesalamine (see Clinical Considerations). There is no information on the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of mesalamine to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for mesalamine and any potential adverse effects on the breastfed child from mesalamine or from the underlying maternal condition.
Clinical Considerations
Advise the caregiver to monitor the breastfed infant for diarrhea.
Data
In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of the N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day (RID 0 to 0.1%) of mesalamine and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.
8.4 Pediatric Use
Safety and effectiveness of mesalamine in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of mesalamine did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia and pancytopenia) in patients who were 65 years or older compared to younger patients taking mesalamine-containing products such as mesalamine. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with mesalamine. In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing mesalamine [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on mesalamine therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue mesalamine if renal function deteriorates while on therapy [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.2)].
-
10 OVERDOSAGE
Mesalamine is an aminosalicylate, and symptoms of salicylate toxicity include nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe salicylate intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) damage. There is no specific antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function. Mesalamine is a pH-dependent delayed-release product and this factor should be considered when treating a suspected overdose.
-
11 DESCRIPTION
Each mesalamine capsule is a delayed- and extended-release dosage form for oral administration. Each capsule contains 0.375 g of mesalamine, USP (5-aminosalicylic acid, 5-ASA), an aminosalicylate. The structural formula of mesalamine is:
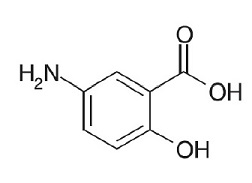
Molecular Weight: 153.14
Molecular Formula: C7H7NO3
Each mesalamine extended-release capsule contains granules composed of mesalamine in a polymer matrix with an enteric coating that dissolves at pH 6 and above.
The inactive ingredients of mesalamine extended-release capsules are: microcrystalline cellulose, hypromellose, colloidal silicon dioxide, ethyl acrylate and methyl methacrylate 2:1 copolymer, magnesium stearate, methacrylic acid and methyl methacrylate 1:1 copolymer, talc, triethyl citrate, titanium dioxide and hypromellose phthalate.
The capsule shell contains titanium dioxide, FD&C Blue 1, FD&C Red 40 and gelatin. The capsule shells are printed with edible black ink containing shellac, black iron oxide and potassium hydroxide.
Meets USP Dissolution Test 5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine (5-ASA) is not fully understood, but appears to be a local anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with ulcerative colitis, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of 5-ASA and its metabolite, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), were studied after a single and multiple oral doses of 1.5 g mesalamine in a crossover study in healthy subjects under fasting conditions. In the multiple-dose period, each subject received mesalamine 1.5 g (four 0.375 g capsules) once daily for 7 consecutive days. Steady state was reached on Day 6 of once daily dosing based on trough concentrations.
After single and multiple doses of mesalamine, peak plasma concentrations were observed at about 4 hours post-dose. At steady state, moderate increases (1.5-fold and 1.7-fold) in systemic exposure (AUC0-24) to 5-ASA and N-Ac-5-ASA were observed when compared with a single-dose of mesalamine.
Pharmacokinetic parameters after a single dose of 1.5 g mesalamine and at steady state in healthy subjects under fasting condition are shown in Table 2.
Table 2: Single Dose and Multiple Dose Mean (±SD) Plasma Pharmacokinetic Parameters of Mesalamine (5-ASA) and N-Ac-5-ASA After 1.5 g Mesalamine Administration in Healthy Subjects
Mesalamine (5-ASA)
Single Dose
(n=24)
Multiple Dosec
(n=24)
AUC0-24 (mcg*h/mL)
AUC0-inf (mcg*h/mL)
Cmax (mcg/mL)
Tmax (h)a
t½ (h)b
11±5
14±5
2.1±1.1
4 (2, 16)
9±7
17±6
-
2.7±1.1
4 (2, 8)
10±8
N-Ac-5-ASA
AUC0-24 (mcg*h/mL)
AUC0-inf (mcg*h/mL)
Cmax (mcg/mL)
Tmax (h)a
t½ (h)b
26±6
51±23
2.8±0.8
4 (4, 12)
12±11
37±9
-
3.4±0.9
5 (2, 8)
14±10
a Median (range); b Harmonic mean (pseudo SD); c after 7 days of treatment
In a separate study (n=30), it was observed that under fasting conditions about 32%±11% (mean±SD) of the administered dose was systemically absorbed based on the combined cumulative urinary excretion of 5-ASA and N-Ac-5-ASA over 96 hours post-dose.
Food Effects
The effect of a high fat meal intake on absorption of mesalamine granules (the same granules contained in mesalamine extended-release capsules) was evaluated in 30 healthy subjects. Subjects received 1.6 g of mesalamine granules in sachet (2 x 0.8 g) following an overnight fast or a high fat meal in a crossover study. Under fed conditions, Tmax for both 5-ASA and N-Ac-5-ASA was prolonged by 4 and 2 hours, respectively. A high fat meal did not affect Cmax for 5-ASA, but a 27% increase in the cumulative urinary excretion of 5-ASA was observed with a high fat meal. The overall extent of absorption of N-Ac-5-ASA was not affected by a high fat meal [see Dosage and Administration (2)].
Distribution
In an in vitro study, at 2.5 mcg/mL, mesalamine and N-Ac-5-ASA are 43±6% and 78±1% bound, respectively, to plasma proteins. Protein binding of N-Ac-5-ASA does not appear to be concentration dependent at concentrations ranging from 1 to 10 mcg/mL.
Elimination
Metabolism
The major metabolite of mesalamine is N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). It is formed by N-acetyltransferase activity in the liver and intestinal mucosa.
Excretion
Following single and multiple doses of mesalamine, the mean half-lives were 9 to 10 hours for 5-ASA, and 12 to 14 hours for N-Ac-5-ASA. Of the approximately 32% of the dose absorbed, about 2% of the dose was excreted unchanged in the urine, compared with about 30% of the dose excreted as N-Ac-5-ASA.
Drug Interaction Studies
In an in vitro study using human liver microsomes, 5-ASA and its metabolite, N-Ac-5-ASA, were shown not to inhibit the major CYP enzymes evaluated (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4). Therefore, mesalamine and its metabolite are not expected to inhibit the metabolism of other drugs that are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary mesalamine was not carcinogenic in rats at doses as high as 480 mg/kg/day, or in mice at 2,000 mg/kg/day. These doses are about 2.6 and 5.4 times the recommended human dose of granulated mesalamine capsules of 1.5 g/day (30 mg/kg if 50 kg body weight assumed or 1,110 mg/m2), respectively, based on body surface area.
Mesalamine was negative in the Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, the sister chromatid exchange assay in the Chinese hamster bone marrow test, and the mouse bone marrow micronucleus test.
No effects on fertility or reproductive performance in male and female rats were observed with oral mesalamine doses up to 320 mg/kg (about 1.7 times the recommended human dose based on body surface area).
13.2 Animal Toxicology and/or Pharmacology
Renal Toxicity
Animal studies with mesalamine (13-week and 26-week oral toxicity studies in rats, and 26-week and 52-week oral toxicity studies in dogs) have shown the kidney to be the major target organ of mesalamine toxicity. Single oral doses of 800 mg/kg (about 2.2 times the recommended human dose, on the basis of body surface area) and 1,800 mg/kg (about 9.7 times the recommended human dose, on the basis of body surface area) of mesalamine were lethal to mice and rats, respectively, and resulted in gastrointestinal and renal toxicity. Oral doses of 40 mg/kg/day (about 0.20 times the human dose, on the basis of body surface area) produced minimal to slight tubular injury, and doses of 160 mg/kg/day (about 0.90 times the human dose, on the basis of body surface area) or higher in rats produced renal lesions including tubular degeneration, tubular mineralization, and papillary necrosis. Oral doses of 60 mg/kg/day (about 1.1 times the human dose, on the basis of body surface area) or higher in dogs also produced renal lesions including tubular atrophy, interstitial cell infiltration, chronic nephritis, and papillary necrosis.
-
14 CLINICAL STUDIES
Two similar, randomized, double-blind, placebo-controlled, multi-center studies were conducted in a total of 562 adult patients in remission from ulcerative colitis. The study populations had a mean age of 46 years (11% age 65 years or older), were 53% female, and were primarily white (92%).
Ulcerative colitis disease activity was assessed using a modified Sutherland Disease Activity Index (DAI), which is a sum of four subscores based on stool frequency, rectal bleeding, mucosal appearance on endoscopy, and physician’s rating of disease activity. Each subscore can range from 0 to 3, for a total possible DAI score of 12.
At baseline, approximately 80% of patients had a total DAI score of 0 or 1. Patients were randomized 2:1 to receive either mesalamine 1.5 g or placebo once daily in the morning for six months. Patients were assessed at baseline, 1 month, 3 months, and 6 months in the clinic, with endoscopy performed at baseline, at end of study, or if clinical symptoms developed. Relapse was defined as a rectal bleeding subscale score of 1 or more and a mucosal appearance subscale score of 2 or more using the DAI. The analysis of the intent-to-treat population was a comparison of the proportions of patients who remained relapse-free at the end of six months of treatment. For the table below (Table 3) all patients who prematurely withdrew from the study for any reason were counted as relapses.
In both studies, the proportion of patients who remained relapse-free at six months was greater for mesalamine than for placebo.
Table 3: Percentage of Ulcerative Colitis Patients Relapse-Free* Through 6 Months in Mesalamine Maintenance Studies
Mesalamine
1.5 g once daily
% (# no relapse/N)
Placebo
% (# no relapse/N)
Difference (95% C.I.)
P-value
Study 1
68% (143/209)
51% (49/96)
17% (5.5, 29.2)
<0.001
Study 2
71% (117/164)
59% (55/93)
12% (0, 24.5)
0.046
*Relapse counted as rectal bleeding score ≥1 and mucosal appearance score ≥2, or premature withdrawal from study.
Examination of gender subgroups did not identify difference in response to mesalamine among these subgroups. There were too few elderly and too few African-American patients to adequately assess difference in effects in those populations.
The use of mesalamine for treating ulcerative colitis beyond six months has not been evaluated in controlled clinical trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Mesalamine extended-release capsules, USP are available as opaque light blue cap / opaque light blue body, hard gelatin capsules size ‘00’ having imprinting “A” on cap in black ink and “255” on body in black ink on either side of a black band filled with light grey to grey color granules and are supplied as follows:
Bottle of 120 capsules with child resistant closure, NDC: 62332-724-35
Bottle of 1000 capsules, NDC: 62332-724-91
Storage:
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Administration
Instruct patients:
Swallow the capsules whole. Do not cut, break, crush or chew the capsules.
Avoid co-administration of mesalamine extended-release capsules with antacids.
Drink an adequate amount of fluids.
Mesalamine extended-release capsules can be taken without regard to meals [see Dosage and Administration (2)].Urine may become discolored reddish-brown while taking mesalamine extended-release capsules when it comes in contact with surfaces or water treated with hypochlorite-containing bleach. If discolored urine is observed, advise patients to observe their urine flow. Report to the healthcare provider only if urine is discolored on leaving the body, before contact with any surface or water (e.g., in the toilet).
Renal Impairment
Inform patients that mesalamine extended-release capsules may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, including NSAIDs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.2)].Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Inform patients of the signs and symptoms of hypersensitivity reactions. Instruct patients to stop taking mesalamine extended-release capsules and report to their healthcare provider if they experience new or worsening symptoms of Acute Intolerance Syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity [see Warnings and Precautions (5.2, 5.3)].
Hepatic Failure
Inform patients with known liver disease of the signs and symptoms of worsening liver function and advise them to report to their healthcare provider if they experience such signs or symptoms [see Warnings and Precautions (5.4)].Severe Cutaneous Adverse Reactions
Inform patients of the signs and symptoms of severe cutaneous adverse reactions. Instruct patients to stop taking mesalamine extended-release capsules and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity [see Warnings and Precautions (5.5)].
Photosensitivity
Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors [see Warnings and Precautions (5.6)].Nephrolithiasis
Instruct patients to drink an adequate amount of fluids during treatment in order to minimize the risk of kidney stone formation and to contact their healthcare provider if they experience signs or symptoms of a kidney stone (e.g., severe side or back pain, blood in the urine) [see Warnings and Precautions (5.7)].Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Drug Interactions (7.3), Use in Specific Populations (8.5)].Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Revised: 12/2023
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MESALAMINE
mesalamine capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62332-724 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 0.375 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) TALC (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE PHTHALATE (31% PHTHALATE, 40 CST) (UNII: G4U024CQK6) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color BLUE (opaque light blue cap) , BLUE (opaque light blue body) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code A;255 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62332-724-35 120 in 1 BOTTLE; Type 0: Not a Combination Product 11/03/2022 2 NDC: 62332-724-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216967 11/03/2022 Labeler - Alembic Pharmaceuticals Inc. (079288842) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(62332-724)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.