ALLERGY RELIEF COMPLETE- diphenhydramine hcl capsule
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by WinCo Foods, LLC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each banded capsule)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Principal Display Panel
WinCo™
FOODSCOMPLETE
Allergy
ReliefDIPHENHYDRAMINE HCl 25 mg/
ANTIHISTAMINE*Compare to the active ingredient
in BENADRYL® AllergyRelieves: Sneezing,
Runny Nose,
Itchy, Watery Eyes
& Itchy Throat24
CapsulesTAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN OR BROKEN OR IF RED BAND AROUND CAPSULE IS BROKEN OR MISSING
DISTRIBUTED BY:
WINCO FOODS, LLC, BOISE, ID 83704*This product is not manufactured or distributed by
JOHNSON & JOHNSON CORPORATION, distributor of
BENADRYL® Allergy.50844 REV1117A19008 WF1803
100% SATISFACTION GUARANTEE
Comments & Questions?
800-824-1706 www.wincofoods.com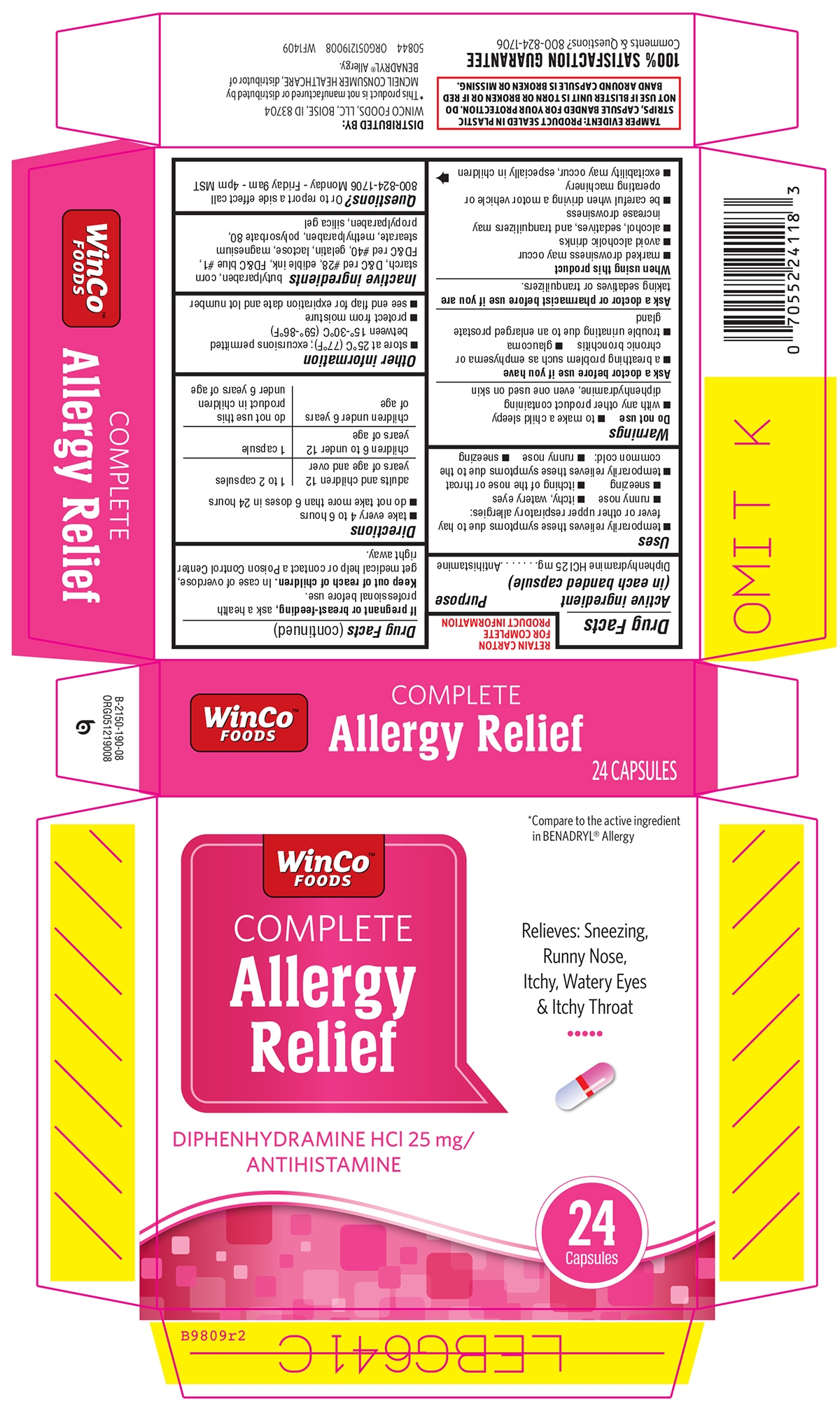
Winco 44-190
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF COMPLETE
diphenhydramine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67091-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color PINK, WHITE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 44;107 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67091-201-24 2 in 1 CARTON 03/15/1990 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 03/15/1990 Labeler - WinCo Foods, LLC (056098817) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(67091-201) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 MANUFACTURE(67091-201) , PACK(67091-201) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(67091-201) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(67091-201)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.