TOPCARE CHILDRENS COLD AND COUGH AND RUNNY NOSE- acetaminophen, chlorpheniramine maleate, and dextromethorphan hydrobromide suspension
TOPCARE by
Drug Labeling and Warnings
TOPCARE by is a Otc medication manufactured, distributed, or labeled by Topco Associates, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert:acetaminophen may cause severe skin reactions.
Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning:if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- to make a child sleepy
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- if your child has ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if your child has
- liver disease
- a breathing problem such as chronic bronchitis
- glaucoma
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if your child is
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dose (seeoverdose warning)
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- excitability may occur, especially in children
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed (see overdose warning)
- shake well before using
- mL = milliliter
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- remove the child protective cap and squeeze your child's dose into the dosing cup
- repeat dose every 4 hours, while symptoms last
- do not give more than 5 times in 24 hours
Weight (lb) Age (yr) Dose (mL) under 36
under 4 years
do not use
36-47
4 to 5 years
do not use unless directed by a doctor
48-95
6 to 11 years
10 mL
Attention:use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions or comments?
-
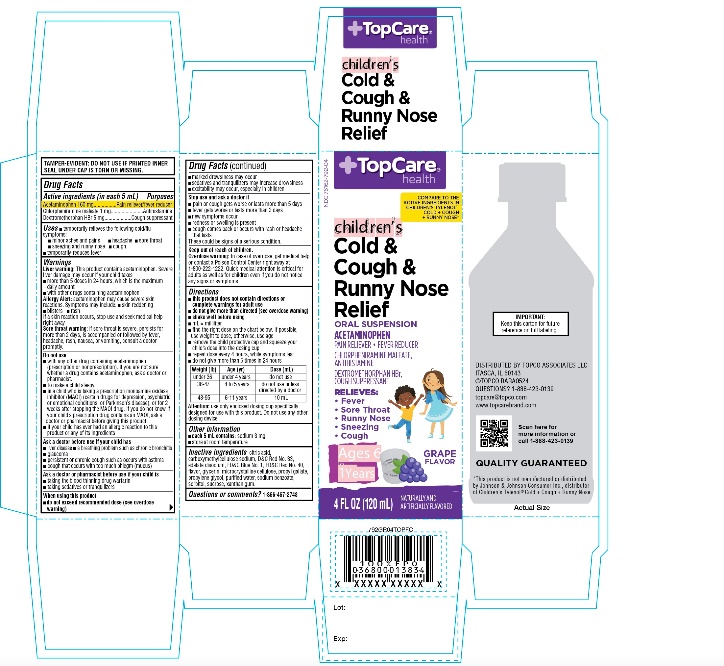
PRINCIPAL DISPLAY PANEL
COMPARE TO THE ACTIVE INGREDIENTS IN CHILDREN’S TYLENOL® COLD + COUGH + RUNNY NOSE*
NDC: 76162-792-04
Children's
Cold & Cough & Runny Nose Relief
ORAL SUSPENSION
ACETAMINOPHEN -PAIN RELIEVER-FEVER REDUCER
CHLORPHENIRAMINE MALEATE -ANTIHISTAMINE
DEXTROMETHORPHAN HBR -COUGH SUPPRESSANTRELIEVES
- Fever
- Sore Throat
- Sneezing
- Runny Nose
- Cough
GRAPE FLAVOR
NATURALLY AND ARTIFICIALLY FLAVORED
Ages 6-11 Years
4 FL OZ (120 mL)
IMPORTANT: Keep this carton for future reference on full labeling.
TAMPER-EVIDENT: DO NOT USE IF PRINTED INNER SEAT UNDER CAP IS TORN OR MISSING.
Distributed by:
*This product is not manufactured or distributed by Johnson & Johnson Consumer Inc., distributor of Children’s Tylenol ®Cold + Cough + Runny Nose.
-
INGREDIENTS AND APPEARANCE
TOPCARE CHILDRENS COLD AND COUGH AND RUNNY NOSE
acetaminophen, chlorpheniramine maleate, and dextromethorphan hydrobromide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76162-792 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 1 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) D&C RED NO. 33 (UNII: 9DBA0SBB0L) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color purple Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76162-792-04 1 in 1 CARTON 08/12/2024 1 120 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/12/2024 Labeler - Topco Associates, LLC (006935977)
Trademark Results [TOPCARE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TOPCARE 98464730 not registered Live/Pending |
Onyeka Tony Osakwe 2024-03-23 |
 TOPCARE 90780839 not registered Live/Pending |
Topco Holdings, Inc. (Cooperative) 2021-06-17 |
 TOPCARE 90105070 not registered Live/Pending |
TopCare Insurance LLC 2020-08-10 |
 TOPCARE 90013975 not registered Live/Pending |
Topco Holdings, Inc. (Cooperative) 2020-06-22 |
 TOPCARE 87799114 5915552 Live/Registered |
Hillcour, Inc. 2018-02-15 |
 TOPCARE 86827212 4997640 Live/Registered |
TOP CARE LAWN SERVICE, INC. 2015-11-20 |
 TOPCARE 85757897 not registered Dead/Abandoned |
TOP-VOX Corporation 2012-10-18 |
 TOPCARE 78524321 3103270 Dead/Cancelled |
TELEDYNE CONTINENTAL MOTORS, INC. 2004-11-30 |
 TOPCARE 78524316 3028770 Dead/Cancelled |
TELEDYNE CONTINENTAL MOTORS, INC. 2004-11-30 |
 TOPCARE 76086414 2473557 Dead/Cancelled |
TELEDYNE CONTINENTAL MOTORS, INC. 2000-07-11 |
 TOPCARE 74536174 not registered Dead/Abandoned |
CIGNA Corporation 1994-06-10 |
 TOPCARE 73668913 1474704 Dead/Cancelled |
STERLING DRUG INC. 1987-06-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.