DOXEPIN HYDROCHLORIDE capsule
Doxepin Hydrochloride by
Drug Labeling and Warnings
Doxepin Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Micro Labs Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DOXEPIN HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for DOXEPIN HYDROCHLORIDE CAPSULES.
DOXEPIN HYDROCHLORIDE capsules, for oral use
Initial U.S. Approval: 1969WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
- Increased risk of suicidal thoughts and behaviors in pediatric and young adults taking antidepressants. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors ( 5.1)
- Doxepin hydrochloride capsules are not approved for use in pediatric patients ( 8.4)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Doxepin hydrochloride capsules are a tricyclic antidepressant (TCA) indicated for the treatment of major depressive disorder (MDD) in adults ( 1).
DOSAGE AND ADMINISTRATION
- Prior to initiating treatment with doxepin hydrochloride capsules, screen patients for a personal or family history of bipolar disorder, mania, or hypomania. ( 2.1)
- Recommended starting oral dosage is 25 mg three times daily or 75 mg once daily. ( 2.2)
- Recommended target total dosage range is between 75 mg/day and 150 mg/day (may be given once daily or in divided doses). ( 2.2)
- Maximum recommended dosage is 100 mg three times daily. ( 2.2)
- Wait at least 14 days after discontinuation of a monoamine oxidase inhibitor (MAOI) before initiating therapy with doxepin hydrochloride capsules. ( 2.3)
- See the Full Prescribing Information for dosage modifications intended to reduce the risk of anticholinergic effects, for strong CYP2D6 inhibitors, and in known CYP2D6 and CYP2C19 poor metabolizers. ( 2.4, 2.5, 2.6)
- When discontinuing doxepin hydrochloride capsules, gradually reduce the dosage until discontinued. ( 2.7)
DOSAGE FORMS AND STRENGTHS
- Capsules: 10 mg, 25 mg, 50 mg, 75 mg, and 100 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Suicidal Thoughts and Behaviors: Monitor for clinical worsening and suicide thoughts and behaviors. Consider changing the therapeutic regimen, including possibly discontinuing doxepin hydrochloride, in patients who are experiencing emergent suicidal thoughts or behaviors. ( 5.1)
- Serotonin Syndrome: Risk increases with concomitant use of other serotonergic drugs. Monitor all patients taking doxepin hydrochloride for the emergence of serotonin syndrome. Discontinue doxepin hydrochloride and any concomitant serotonergic agents immediately and initiate supportive treatment if serotonin syndrome occurs. ( 5.2, 7)
- Angle-Closure Glaucoma: Avoid use of doxepin hydrochloride in patients with untreated anatomically narrow angles. ( 5.3)
- Sedation and Driving Risks: Because doxepin hydrochloride can cause sedation, warn patients against driving a car or operating dangerous machinery while taking doxepin hydrochloride. ( 5.4)
- Activation of Mania or Hypomania: Prior to initiating antidepressant therapy, screen for bipolar disorder. doxepin hydrochloride is not approved for use in treating bipolar depression. ( 5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are somnolence, dry mouth, dizziness, constipation and fatigue. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Micro Labs USA, Inc. at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Serotonergic Drugs: Monitor patients for signs and symptoms of serotonin syndrome, particularly during treatment initiation and dosage increases. If serotonin syndrome occurs, consider discontinuation of doxepin hydrochloride and/or concomitant serotonergic drugs. ( 5.2, 7)
- Strong CYP2D6 Inhibitors: Concomitant use of TCAs with drugs that can inhibit CYP2D6 may require lower dosages for the TCA or the other drug, and monitor TCA plasma levels. ( 7)
- Carbamazepine: Monitor doxepin plasma concentrations and increase doxepin hydrochloride dosage in patients taking carbamazepine. ( 7)
- Cimetidine: Monitor doxepin plasma concentrations and consider reducing the doxepin hydrochloride dosage in patients taking cimetidine. ( 7)
- Alcohol: Avoid concomitant use. ( 7)
- CNS Depressants: Dosage reduction may be needed based on clinical response and tolerability. ( 7)
- Tolazamide: Monitor glucose levels and reduce the doxepin hydrochloride dosage as appropriate. ( 7)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Neonates exposed to TCAs, including doxepin hydrochloride, late in the third trimester have developed poor adaptation (respiratory distress, temperature instability, feeding difficulty, hypotonia, irritability). Monitor neonates who were exposed to doxepin hydrochloride in the third trimester of pregnancy for poor neonatal adaptation syndrome. ( 8.1)
- Lactation: Breastfeeding not recommended. ( 8.2)
- Geriatric Use: May cause confusion and oversedation. ( 8.5)
- CYP2C19 and CYP2D6 Poor Metabolizers: Increased risk of doxepin hydrochloride-associated adverse reactions. ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Screen for Bipolar Disorder Prior to Starting Doxepin Hydrochloride Capsules
2.2 Recommended Dosage
2.3 Switching Patients to or from a Monoamine Oxidase Inhibitor
2.4 Dosage Modifications Intended to Reduce the Risk of Anticholinergic Effects
2.5 Dosage Modifications for Strong CYP2D6 Inhibitors
2.6 Dosage Modifications in Known CYP2D6 and CYP2C19 Poor Metabolizers
2.7 Discontinuation of Doxepin Hydrochloride Capsules Treatment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 Serotonin Syndrome
5.3 Angle-Closure Glaucoma
5.4 Sedation and Driving Risks
5.5 Activation of Mania or Hypomania
5.6 Risk of Seizures
5.7 Psychosis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Use in Genomic Subgroups
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increase the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Doxepin hydrochloride capsules are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Screen for Bipolar Disorder Prior to Starting Doxepin Hydrochloride Capsules
Prior to initiating treatment with doxepin hydrochloride capsules, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage
The recommended starting oral dosage for doxepin hydrochloride capsule is 25 mg three times daily or 75 mg once daily. The recommended target total oral dosage range for doxepin hydrochloride capsule is between 75 mg/day and 150 mg/day (may be given once daily or in divided doses). The maximum recommended oral dosage for doxepin hydrochloride capsule is 100 mg three times daily.
2.3 Switching Patients to or from a Monoamine Oxidase Inhibitor
Wait at least 14 days after discontinuation of a monoamine oxidase inhibitor (MAOI) before initiating therapy with doxepin hydrochloride capsules [see Contraindications (4), Warnings and Precautions (5.2), and Drug Interactions (7)] .
Wait at least 14 days after discontinuation of doxepin hydrochloride capsules before initiating therapy with an MAOI [see Contraindications (4), Warnings and Precautions (5.2), and Drug Interactions (7)].
2.4 Dosage Modifications Intended to Reduce the Risk of Anticholinergic Effects
If anticholinergiceffects (e.g., dry mouth, blurred vision, constipation) develop, reduce the doxepin hydrochloride capsules dosage [see Adverse Reactions (6.1)].
2.5 Dosage Modifications for Strong CYP2D6 Inhibitors
Reducethe doxepin hydrochloride capsules dosage based on doxepin plasma concentrations when used concomitantly with strong CYP2D6 inhibitors [see Drug Interactions (7)].
2.6 Dosage Modifications in Known CYP2D6 and CYP2C19 Poor Metabolizers
Reducethe doxepin hydrochloride capsules dosage based on doxepin plasma concentrations in patients who are known CYP2D6 and CYP2C19 poormetabolizers [see Use in Specific Populations (8.7)].
2.7 Discontinuation of Doxepin Hydrochloride Capsules Treatment
Whendiscontinuing doxepin hydrochloride capsules, gradually reduce the dosage until discontinued [see Adverse Reactions (6)] .
-
3 DOSAGE FORMS AND STRENGTHS
Capsules:
- Doxepin hydrochloride capsules USP, 10 mg:size “4” hard gelatin capsule with buff opaque cap and buff opaque body, imprinted “10” on cap with black ink and plain body filled with white to of white powder.
- Doxepin hydrochloride capsules USP, 25 mg:size "3" hard gelatin capsule with ivory opaque cap and white opaque body, imprinted "25" on cap with black ink and plain body filled with white to off white powder.
- Doxepin hydrochloride capsules USP, 50 mg:size "3" hard gelatin capsule with ivory opaque cap and ivory opaque body, imprinted "50" on cap with black ink and plain body filled with white to off white powder.
- Doxepin hydrochloride capsules USP, 75 mg:size "2" hard gelatin capsule with brite lite green opaque cap and brite lite green opaque body, imprinted "75" on cap with black ink and plain body filled with white to off white powder.
- Doxepin hydrochloride capsules USP, 100 mg:size "1" hard gelatin capsule with brite lite green opaque cap and white opaque body, imprinted "100" on cap with black ink and plain body filled with white to off white powder.
Active ingredients in the capsules include: 10 mg, 25, mg, 50 mg, 75 mg, and 100 mg of doxepin.
-
4 CONTRAINDICATIONS
Doxepin hydrochloride capsules are contraindicated in patients:
- With hypersensitivity to doxepin (hypersensitivity reactions have included tongue edema and urticaria). The possibility of cross sensitivity with other dibenzoxepines should be kept in mind.
- With glaucoma [see Warnings and Precautions (5.3)].
- With current or past urinary retention [see Adverse Reactions (6.1)].
- Taking MAOIs, or within 14 days of stopping MAOIs (including the MAOIs linezolid or intravenous methylene blue) because of an increased risk of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs including tricyclic antidepressants and other antidepressant classes that included approximately 77,000 adult patients and 4,500 pediatric patients (doxepin hydrochloride is not approved for use in pediatric patients), the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
Table 1: Risk Differences of the Number of Patients of Suicidal Thoughts and Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
Age Range
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1,000 Patients Treated
Increases Compared to Placebo
< 18 years old
14 additional patients
18 to 24 years old
5 additional patients
Decreases Compared to Placebo
25 to 64 years old
1 fewer patient
≥ 65 years old
6 fewer patients
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all doxepin hydrochloride-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of doxepin hydrochloride therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the health care provider. Consider changing the therapeutic regimen, including possibly discontinuing doxepin hydrochloride, in patients who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
Tricyclic antidepressants,including doxepin hydrochloride, can precipitate serotonin syndrome, a potentially life-threatening condition. This risk is increased with concomitantuse of other serotonergic drugs (e.g., other tricyclic antidepressants, SSRIs, serotonin norepinephrine reuptake inhibitors, triptans,tetracyclic antidepressants, opioids), lithium, tryptophan, buspirone, and St. John’s Wort) and with drugs that impair metabolism of serotonin (e.g., MAOIs intended totreat psychiatric disorders and others, such as linezolid or intravenous methylene blue) [see Drug Interactions (7)] .
Serotonin syndrome symptomsmay include mental status changes (e.g., confusion, agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia,labile blood pressure, hyperthermia, diaphoresis, and flushing), neuromuscular abnormalities (e.g., tremor, rigidity, clonus, and hyperreflexia), seizures and gastrointestinalsigns and symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotoninsyndrome.
The concomitantuse of doxepin hydrochloride with MAOIs is contraindicated. The use of doxepin hydrochloride within 14 days of discontinuing treatmentwith an MAOI intended to treat psychiatric disorders is contraindicated. Starting doxepin hydrochloride in a patient who is being treated withan MAOI such as linezolid or intravenous methylene blue is contraindicated. No reports involved the administration of methylene blue by other routes (such as oral or local tissue injection). If it isnecessary to initiate treatment with a MAOI such as linezolid or intravenous methylene blue in a patient taking doxepin hydrochloridecapsules, discontinue doxepin hydrochloride capsules before initiating treatment with the MAOI [see Dosage and Administration (2.4) and Drug Interactions (7)] .
Monitorall patients taking doxepin hydrochloride capsules for the emergence of serotonin syndrome. Discontinue doxepin hydrochloride treatment and any concomitantserotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use ofdoxepin hydrochloride with other serotonergic drugs (besides MAOIs which are contraindicated) is clinically warranted, inform patients of the increased risk forserotonin syndrome and monitor for symptoms.
5.3 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including doxepin hydrochloride may trigger an angle closure glaucoma attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Doxepin hydrochloride capsules are contraindicated in patients with glaucoma. Avoid use of doxepin hydrochloride capsules in patients with untreated anatomically narrow angles.
5.4 Sedation and Driving Risks
Because doxepin hydrochloride can cause sedation, warn patients of the risk of sedation and caution patients against driving a car or operating dangerous machinery while taking doxepin hydrochloride capsules. Also caution patients that their response to alcohol may be potentiated.
Sedating drugs, including doxepin hydrochloride, may cause oversedation in geriatric patients.
5.5 Activation of Mania or Hypomania
In patientswith bipolar disorder, treating MDD with doxepin hydrochloride may precipitate a mixed/manic episode. Prior to initiating treatment with doxepin hydrochloride capsules,screen patients for any personal or family history of bipolar disorder, mania, or hypomania. Doxepin hydrochloride is not approved for use intreating bipolar depression.
5.6 Risk of Seizures
Caution should be used when doxepin hydrochloride is given to patients with a history of seizure disorder, because this drug may lower the seizure threshold. Patients with a history of seizures should be monitored during doxepin hydrochloride use to identify recurrence of seizures or increase in frequency of seizures.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Angle-Closure Glaucoma [see Warnings and Precautions (5.3)]
- Sedation and Driving Risks [see Warnings and Precautions (5.4)]
- Activation of Mania or Hypomania [see Warnings and Precautions (5.5)]
- Risk of Seizures [see Warnings and Precautions (5.6)]
- Psychosis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions (≥ 2% of doxepin hydrochloride-treated patients) in 1,635 doxepin hydrochloride-treated patients with MDD in clinical trials included somnolence (17%), dry mouth (15%), dizziness (6%), constipation (5%), fatigue (5%), blurred vision (3%), tachycardia (3%), hypotension (3%), insomnia (2%), tremor (2%), nausea (2%), hyperhidrosis (2%), and increased weight (2%).
Other Adverse Reactions Observed in Clinical Trials
Other adverse reactions that occurred at an incidence of < 2% in patients treated with doxepin hydrochloride in clinical trials were:
- Ear and Labyrinth Disorders: Tinnitus.
- Gastrointestinal Disorders: Diarrhea, dyspepsia, vomiting.
- General Disorders and Administration Site Conditions: Asthenia, edema, chills.
- Metabolism and Nutrition Disorders: Decreased appetite.
- Nervous System Disorders: Ataxia, paresthesia, headache, extrapyramidal disorder.
- Psychiatric Disorders: Agitation, confusional state, libido decreased.
- Pulmonary Disorders: Asthma exacerbation.
- Renal and Urinary Disorders: Urinary retention.
- Reproductive System and Breast Disorders: Breast enlargement.
- Skin & Subcutaneous Tissue Disorders: Rash, pruritus.
- Vascular Disorders: Flushing.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of doxepin hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Agranulocytosis, leukopenia, thrombocytopenia, eosinophilia, purpura.
- Cardiac Disorders: Conduction disorder, arrhythmia.
- Endocrine Disorders: Inappropriate antidiuretic hormone secretion.
- Eye Disorders: Angle-closure glaucoma, mydriasis.
- Gastrointestinal Disorders: Aphthous stomatitis, abdominal pain upper.
- General Disorders and Administration Site Conditions: Facial edema, hyperpyrexia.
- Hepatobiliary Disorders: Jaundice.
- Investigations: Blood glucose increased.
- Nervous System Disorders: Hypoesthesia, dysgeusia, convulsion, tardive dyskinesia, serotonin syndrome.
- Psychiatric Disorders: Hallucination, disorientation.
- Reproductive System and Breast Disorders: Testicular swelling, gynecomastia, galactorrhea.
- Skin and Subcutaneous Tissue Disorders: Photosensitivity reaction, tongue edema, alopecia, urticaria.
- Vascular Disorders: Hypertension.
Withdrawal syndrome occurred after stopping doxepin hydrochloride [see Drug Abuse and Dependence (9.3)].
The following adverse reaction has been reported with use with other tricyclic antidepressants: decreased blood glucose.
-
7 DRUG INTERACTIONS
Table 2 describe the clinically significant drug interactions of doxepin hydrochloride with other drugs or classes.
Table 2: Clinically Significant Drug Interactions with Doxepin Hydrochloride
Monoamine Oxidase Inhibitors
Prevention or Management
Doxepin hydrochloride is contraindicated in patients taking monoamine oxidase inhibitors (MAOIs), including MAOIs such as linezolid or intravenous methylene blue. The use of doxepin hydrochloride within 14 days of discontinuation of an MAOI or the use of MAOI within 14 days of discontinuation of doxepin hydrochloride is contraindicated. Starting doxepin hydrochloride in a patient who is being treated with an MAOI is contraindicated.
Clinical Effect(s)
Concomitant use of doxepin hydrochloride and MAOIs increases the risk of serotonin syndrome [see Warnings and Precautions (5.2)] .
Other Serotonergic Drugs (Besides MAOIs)
Prevention or Management
Monitor patients for signs and symptoms of serotonin syndrome, particularly during treatment initiation and dosage increases. If serotonin syndrome occurs, consider discontinuation of doxepin hydrochloride and/or concomitant serotonergic drugs [see Warnings and Precautions (5.2)].
Mechanism and Clinical Effect(s)
Concomitant use of doxepin hydrochloride with other serotonergic drugs increases the risk of serotonin syndrome [see Warnings and Precautions (5.2)].
Strong CYP2D6 Inhibitors
Prevention or Management
Monitor doxepin plasma concentrations and reduce the doxepin hydrochloride dosage or the strong CYP2D6 inhibitor as appropriate [see Dosage and Administration (2.5)].
Mechanism and Clinical Effect(s)
Concomitant use of doxepin hydrochloride with strong CYP2D6 inhibitors may increase the exposures of doxepin [see Clinical Pharmacology (12.3)] which may increase the risk of doxepin hydrochloride related adverse reactions [see Warnings and Precautions (5) and Adverse Reactions (6)] .
Examples
See www.fda.gov/CYPandTransporterInteractingDrugs for examples of strong CYP2D6 Inhibitors.
Carbamazepine
Prevention or Management
Monitor doxepin plasma concentrations and consider increasing the doxepin hydrochloride dosage in patients taking carbamazepine.
Mechanism and Clinical Effect(s)
Concomitant use of carbamazepine with doxepin hydrochloride decreases the exposure of doxepin [see Clinical Pharmacology (12.3)] which could lead to reduced treatment effect.
Cimetidine
Prevention or Management
Monitor doxepin plasma concentrations and consider reducing the doxepin hydrochloride dosage in patients taking cimetidine.
Mechanism and Clinical Effect(s)
Concomitant use of doxepin hydrochloride with cimetidine may increase the exposures of doxepin [see Clinical Pharmacology (12.3)] which may increase the risk of doxepin hydrochloride-related anticholinergic effects (e.g., dry mouth, blurred vision, constipation) [see Adverse Reactions (6.1)] .
Alcohol
Prevention or Management
Avoid concomitant use with alcohol.
Mechanism and Clinical Effect(s)
doxepin hydrochloride may potentiate the sedative effects of alcohol [see Warnings and Precautions (5.4)].
CNS Depressants
Prevention or Management
Dosage reduction of doxepin hydrochloride and/or the CNS depressant may be needed based on clinical response and tolerability.
Mechanism and Clinical Effect(s)
When concomitantly administered with doxepin hydrochloride, the sedative effects of CNS depressant may be potentiated [see Warnings and Precautions (5.4)].
Tolazamide
Prevention or Management
Monitor glucose levels and reduce the doxepin hydrochloride dosage as appropriate.
Clinical Effect(s)
Doxepin hydrochloride may cause severe hypoglycemia when concomitantly used with tolazamide.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including doxepin hydrochloride, during pregnancy. Health care providers are encouraged to advise patients to register by calling the National Pregnancy Registry for Antidepressants 1-866-961-2388 or visiting online at https://womensmentalhealth.org/clinical-and-researchprograms/pregnancyregistry/antidepressants.
Risk Summary
Available data from published epidemiological studies and postmarketing reports have not established an increased risk for major birth defects or miscarriage with doxepin hydrochloride use (see Data).There are risks (see Clinical Considerations):
- To the mother associated with untreated depression in pregnancy.
- Poor neonate adaptation from exposure to tricyclic antidepressants (TCAs), including doxepin hydrochloride, during the third trimester of pregnancy .
Animal reproduction toxicity of doxepin has not been fully characterized.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryofetal Risk
Women who discontinue antidepressants during pregnancy are more likely to experience a relapse of MDD than women who continue antidepressants. This finding is from a prospective longitudinal study of 201 pregnant women with a history of MDD who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated MDD when considering discontinuation of doxepin hydrochloride drugs during pregnancy and the postpartum period.
Fetal/Neonatal Adverse Reactions
Neonates previously exposed to TCAs, including doxepin hydrochloride, late in the third trimester during pregnancy have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These findings are consistent with either direct toxic effects of TCAs or possibly a drug discontinuation syndrome. Monitor neonates who were exposed to doxepin hydrochloride in the third trimester of pregnancy for poor neonatal adaptation syndrome.
Data
Human Data:Published epidemiological studies of pregnant women exposed to TCAs, including doxepin hydrochloride, have not established an association with major birth defects, miscarriage, or adverse maternal outcomes. Methodological limitations of these observational studies include small sample size and lack of adequate controls.
8.2 Lactation
Risk Summary
Data from published literature report the presence of doxepin and nordoxepin in human milk. There are reports of excessive sedation, respiratory depression, poor suckling and swallowing and hypotonia in breastfed infants exposed to doxepin at doses used to treat MDD. There are no data on the effects of doxepin on milk production.
Because of the potential for serious adverse reactions, including excess sedation and respiratory depression in a breastfed infant, advise patients that breastfeeding is not recommended during doxepin hydrochloride treatment.
8.4 Pediatric Use
The safety and effectiveness of doxepin hydrochloride in pediatric patients have not been established.
Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric patients [see Warnings and Precautions (5.1)] .
8.5 Geriatric Use
Clinical studies of doxepin hydrochloride did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
Sedating drugs, including doxepin hydrochloride, may cause confusion and oversedation in geriatric patients. The recommended starting doxepin hydrochloride dosage in geriatric patients is generally lower than those of younger adult patients .
8.6 Hepatic Impairment
The effect of hepatic impairment (HI) on the pharmacokinetics of doxepin has not been studied. Doxepin is primarily metabolized in the liver. Doxepin hydrochloride-treated patients with HI may have a greater systemic doxepin exposure than those with normal liver function. Consider obtaining doxepin concentrations in patients with HI and modifying the dosage as appropriate.
8.7 Use in Genomic Subgroups
The recommended doxepin hydrochloride dosage in CYP2C19 and CYP2D6 poor metabolizers is lower than the recommended dosage in CYP2C19 and CYP2D6 normal metabolizers [see Dosage and Administration (2.6)].
According to the literature, doxepin is primarily metabolized by CYP2D6 and/or CYP2C19; thus, the use of doxepin hydrochloride in CYP2D6 and/or CYP2C19 poor metabolizers will likely result in higher doxepin exposures and an increased risk of doxepin hydrochloride-associated adverse reactions.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Doxepin hydrochloride capsules contains doxepin, which is not a controlled substance.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt cessation of doxepin hydrochloride capsules after prolonged administration can result in withdrawal symptoms, which is indicative of physical dependence.
-
10 OVERDOSAGE
Signs, Symptoms, and Complications of Doxepin Hydrochloride Overdose
Serious manifestations of tricyclic antidepressant (TCA) overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Deaths may occur from overdosage with TCAs, including doxepin hydrochloride. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of TCA toxicity. A maximal limb-lead QRS duration of ≥ 0.1 seconds may be the best indication of the TCA overdose severity.
Signs and symptoms of TCA toxicity develop rapidly after TCA overdose. Other signs of TCA overdose may include confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, or hyperpyrexia. There are reports of patients succumbing to fatal dysrhythmia late after TCA overdose.
Management of Overdose
The following are recommendations for the management of a doxepin hydrochloride overdose. Contact the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
With a doxepin hydrochloride overdose, obtain an ECG and immediately initiate cardiac monitoring in the hospital. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS depression, respiratory depression, hypotension, cardiac dysrhythmias, conduction blocks, and seizures is recommended. If signs of toxicity occur during this period, extended monitoring is recommended.
Monitoring of plasma doxepin levels should not guide doxepin hydrochloride overdose management.
Cardiovascular Toxicity Management:Intravenous sodium bicarbonate should be administered to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate to intravenous sodium bicarbonate therapy, hyperventilation may also be used. With concomitant use of hyperventilation and sodium bicarbonate therapy frequently monitor pH and pCO 2. A pH > 7.6 or a pCO 2< 20 mm Hg is undesirable. Dysrhythmias unresponsive to intravenous sodium bicarbonate therapy/hyperventilation may respond to lidocaine therapy. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide) in the setting of TCA overdose. Hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in TCA overdose due to high tissue and protein binding of doxepin.
CNS Toxicity Management:In patients with TCA overdose who have CNS depression, early intubation is recommended because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, propofol). Avoid use of physostigmine to treat TCA overdose.
-
11 DESCRIPTION
Doxepin is a tricyclic antidepressant.
The molecular formula of doxepin hydrochloride, USP is C 19H 21NOHCl with a molecular weight of 315.84. It is a white crystalline solid soluble in water, lower alcohols and chloroform. Doxepin is a dibenzoxepin derivative. Specifically, it is an isomeric mixture of: 1-Propanamine, 3-dibenz[ b, e]oxepin-11(6 H)ylidene- N, N-dimethyl-, hydrochloride. It structural formula of doxepin is shown below.
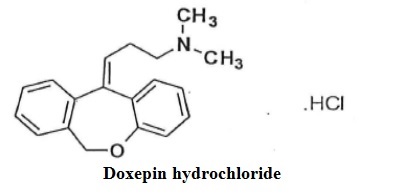
Doxepin hydrochloride capsules, USP are for oral administration.
Active ingredients for the capsules include: 10 mg, 25 mg, 50 mg, 75 mg, and 100 mg of doxepin (equivalent to 11.30 mg, 28.26 mg, 56.52 mg, 84.78 mg, and 113.05 mg of doxepin hydrochloride, respectively).
Inactive ingredients: magnesium stearate, pregelatinised starch, silicified microcrystalline cellulose and sodium lauryl sulfate.
The hard gelatin capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 10 mg, 25 mg and 50 mg capsule shells contains FD&C Yellow No. 5 and FD&C Red 40; and 75 mg and 100 mg capsules shells contains D&C Yellow 10 and FD&C Green 3.
The imprinting black ink contains black iron oxide, potassium hydroxide and shellac.
Meets USP Dissolution Test 3.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of the doxepin hydrochloride in the treatment of MDD in adult patients is not well understood.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of doxepin have not been fully characterized.
12.3 Pharmacokinetics
Absorption
In healthy volunteers, a single oral doxepin hydrochloride dose of 75 mg resulted in peak plasma doxepin concentrations that ranged from 8.8 ng/mL to 45.8 ng/mL (mean 26.1 ng/mL). Peak levels were reached between 2 and 4 hours (mean 2.9 hours) after doxepin hydrochloride administration. Peak levels for the primary active metabolite N-desmethyldoxepin (nordoxepin) ranged from 4.8 ng/mL to 14.5 ng/mL (mean 9.7 ng/mL) and were achieved between 2 and 10 hours after doxepin hydrochloride administration.
Distribution
The mean apparent volume of distribution for doxepin was approximately 20 L/kg. The protein binding for doxepin was approximately 76%.
Elimination
In healthy volunteers, the plasma elimination half-life of doxepin ranged from 8 to 24 hours (mean 17 hours). The half-life of nordoxepin ranged from 33 to 80 hours (mean 51 hours). The mean plasma clearance for doxepin was approximately 0.84 L/hour/kg.
Metabolism
After oral doxepin hydrochloride capsules administration, approximately 55% to 87% of doxepin undergoes first-pass metabolism in the liver, forming the primary active metabolite nordoxepin. Metabolic pathways of doxepin include demethylation, N-oxidation, hydroxylation and glucuronide formation.
Excretion
Doxepin is excreted primarily in the urine, mainly as its metabolites, either free or in conjugate form.
Specific Populations
Patients with Hepatic Impairment:Specific clinical studies have not been performed to evaluate the pharmacokinetics of doxepin in patients with hepatic impairment. Patients with hepatic impairment may have a greater systemic doxepin exposure than those with normal liver function [see Use in Specific Populations (8.6)].
Patients with Renal Impairment:The extent of renal excretion of doxepin is unknown. Specific clinical studies have not been performed to evaluate the pharmacokinetics of doxepin in patients with renal impairment compared to those with normal renal function.
Drug Interaction Studies
Carbamazepine: After concomitant use of doxepin hydrochloride and carbamazepine, the combined exposure of doxepin and nordoxepin (12 hours after the last dose) was decreased by 55% compared to that after the use of doxepin hydrochloride alone [see Drug Interactions (7)].
Strong CYP2D6 Inhibitors: CYP2D6 contributes to the metabolism of doxepin and concomitant use of doxepin hydrochloride with strong CYP2D6 inhibitors may increase doxepin exposure [see Drug Interactions (7)].
Cimetidine:Cimetidine is a non-specific inhibitor of CYP1A2, 2C19, 2D6, and 3A4. When cimetidine 300 mg twice daily was administered concomitantly with a single 6 mg dose of another oral doxepin product, there was approximately a 2-fold increase in doxepin C maxand AUC compared to doxepin without cimetidine [see Drug Interactions (7)] .
CYP2D6 Substrates:Concomitant use of doxepin hydrochloride and other CYP2D6 substrates may have impact on the plasma doxepin concentrations. The clinical significance of this possible impact is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of doxepin in animals has not been fully characterized.
Mutagenesis
The mutagenetic potential of doxepin in animals has not been fully characterized.
Impairment of Fertility
Doxepin had no effect on female fertility in rats at oral doses up to 25 mg/kg/day (1.6x the human dose of 150 mg/day on a mg/m 2basis for a 60 kg human).
Insemination and conception were reduced in untreated female rats mated with male rats administered doxepin at 25 mg/kg/day for a period of ≥ 7 months.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Doxepin hydrochloride capsules, USP are available as capsules containing doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg or 100 mg of doxepin.
Doxepin hydrochloride capsules USP, 10 mgare supplied as size "4" hard gelatin capsule with buff opaque cap and buff opaque body, imprinted "10" on cap with black ink and plain body filled with white to off white powder. They are available as follows:
Bottles of 100 with child-resistant closure: NDC: 42571-420-01
Bottles of 1000: NDC: 42571-420-13
Doxepin hydrochloride capsules USP, 25 mgare supplied as size "3" hard gelatin capsule with ivory opaque cap and white opaque body, imprinted "25" on cap with black ink and plain body filled with white to off white powder. They are available as follows:
Bottles of 100 with child-resistant closure: NDC: 42571-421-01
Bottles of 1000: NDC: 42571-421-13
Doxepin hydrochloride capsules USP, 50 mgare supplied as size "3" hard gelatin capsule with ivory opaque cap and ivory opaque body, imprinted "50" on cap with black ink and plain body filled with white to off white powder. They are available as follows:
Bottles of 100 with child-resistant closure: NDC: 42571-422-01
Bottles of 1000: NDC: 42571-422-13
Doxepin hydrochloride capsules USP, 75 mgare supplied as size "2" hard gelatin capsule with brite lite green opaque cap and brite lite green opaque body, imprinted "75" on cap with black ink and plain body filled with white to off white powder. They are available as follows:
Bottles of 100 with child-resistant closure: NDC: 42571-423-01
Bottles of 1000: NDC: 42571-423-13
Doxepin hydrochloride capsules USP, 100 mgare supplied as size "1" hard gelatin capsule with brite lite green opaque cap and white opaque body, imprinted "100" on cap with black ink and plain body filled with white to off white powder. They are available as follows:
Bottles of 100 with child-resistant closure: NDC: 42571-424-01
Bottles of 1000: NDC: 42571-424-13
Store doxepin hydrochloride capsules at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. Keep container tightly closed.
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal thoughts and behaviors, especially early during doxepin hydrochloride capsules treatment and when the dosage is increased or decreased, and instruct them to report suicidal thinking and behavior to their health care provider [see Warnings and Precautions (5.1)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome particularly with the concomitant use of doxepin hydrochloride capsules and other serotonergic drugs (e.g., other TCAs, SSRIs, SNRIs, triptans, opioids), lithium, tryptophan, buspirone, and St. John’s Wort and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid) [see Warnings and Precautions (5.2), Drug Interactions (7)]. Instruct patients to contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Angle-Closure Glaucoma
Advise patients that taking doxepin hydrochloride capsules can cause pupillary dilation, which in susceptible individuals, can trigger angle closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.3)] .
Effects on Driving and Operating Heavy Machinery
Inform patients that doxepin hydrochloride capsules can cause sedation and caution them against driving a car or operating dangerous machinery while taking doxepin hydrochloride capsules [see Warnings and Precautions (5.4)] .
Activation of Mania or Hypomania
Advise patients to observe for signs of mania/hypomania activation and instruct them to report such symptoms to the healthcare provider.
Drug Interactions
Inform patients that the use of doxepin hydrochloride capsules and certain other drugs increases the risk of doxepin hydrochloride capsules-associated adverse reactions or alternatively lower doxepin hydrochloride capsules effectiveness. Instruct patients to inform their healthcare provider about all the drugs that they are taking before taking doxepin hydrochloride capsules.
Alcohol Use
Advise patients to avoid the use of alcohol while taking doxepin hydrochloride capsules [see Drug Interactions (7)].
Pregnancy
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to doxepin hydrochloride capsules during pregnancy. Advise women to notify their healthcare provider if they become pregnant or intend to become pregnant during doxepin hydrochloride capsules treatment.
Advise pregnant women that doxepin hydrochloride capsules use late in pregnancy may increase the risk for neonatal complications requiring prolonged hospitalization, respiratory support, or tube feeding [see Use in Specific Populations (8.1)].
Lactation
Advise patients that breastfeeding is not recommended during doxepin hydrochloride capsules treatment [see Use in Specific Populations (8.2)] .
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA, Inc.
Somerset, NJ 08873
Rev. 07/2025
-
MEDICATION GUIDE
Doxepin Hydrochloride
(dox' e pin hye'' droe klor' ide)
Capsules, USP
for oral useWhat is the most important information I should know about doxepin hydrochloride capsules?
Doxepin hydrochloride capsules can cause serious side effects, including:
Increased risk of suicidal thoughts and actions.Doxepin hydrochloride capsules and other antidepressant medicines may increase the risk of suicidal thoughts and actions in people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. Doxepin hydrochloride capsules are not for use in children.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your health care provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings or if you develop suicidal thoughts or actions.
- Keep all follow-up visits with your health care provider as scheduled. Call your health care provider between visits as needed, especially if you have concerns about symptoms.
o suicide attempts o thoughts about suicide or dying o acting aggressive, being angry, or violent o acting on dangerous impulses o new or worse depression o new or worse anxiety o panic attacks o feeling very agitated or restless o new or worse irritability o trouble sleeping o an extreme increase in activity or talking (mania) o other unusual changes in behavior or mood See “What are the possible side effects of doxepin hydrochloride capsules?”for more information about side effects. What are doxepin hydrochloride capsules?
Doxepin hydrochloride capsules are a prescription medicine used to treat adults with a certain type of depression called major depressive disorder (MDD).
It is not known if doxepin hydrochloride capsules are safe and effective for use in children.Do not take doxepin hydrochloride capsules if you:
- are allergic to doxepin, or any of the ingredients in doxepin hydrochloride capsules. See the end of this Medication Guide for a complete list of ingredients in doxepin hydrochloride capsules
- have glaucoma
- have or have had trouble urinating
- are taking, or have stopped taking within the last 14 days, a medicine called a Monoamine Oxidase Inhibitor (MAOI), including the antibiotic linezolid or intravenous methylene blue
- Ask your health care provider or pharmacist if you are not sure if you are taking an MAOI, including the antibiotic linezolid or intravenous methylene blue
-
Do not start taking an MAOI for at least 14 days after you stop treatment with doxepin hydrochloride capsules
Before taking doxepin hydrochloride capsules, tell your health care provider about all your medical conditions, including if you:
- have, or have a family history of bipolar disorder, mania, or hypomania
- have or had depression, suicidal thoughts or behavior
- have kidney or liver problems
- have or had seizures or convulsions
- are pregnant or plan to become pregnant. Taking doxepin hydrochloride capsules during your third trimester of pregnancy may harm your unborn baby. Tell your health care provider if you become pregnant or think you may be pregnant during treatment with doxepin hydrochloride capsules
- Babies born to mothers who take certain medicines, including doxepin hydrochloride capsules, during the third trimester of pregnancy may have symptoms of sedation, such as breathing problems, sluggishness, low muscle tone, feeding problems, and withdrawal symptoms. Talk to your health care provider about the risks to your unborn or newborn baby if you take doxepin hydrochloride capsules during pregnancy
- There is a pregnancy registry for women who are exposed to doxepin hydrochloride capsules during pregnancy. The purpose of this registry is to collect information about the health of women exposed to doxepin hydrochloride capsules and their babies. If you become pregnant during treatment with doxepin hydrochloride capsules, talk to your health care provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-866-961-2388 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/
- are breastfeeding or plan to breastfeed. Doxepin hydrochloride can pass into your breast milk and harm your baby. Do not breastfeed during treatment with doxepin hydrochloride capsules. Talk to your health care provider about the best way to feed your baby during treatment with doxepin hydrochloride capsules
Doxepin hydrochloride capsules and other medicines may affect each other causing possible serious side effects. Doxepin hydrochloride capsules may affect the way other medicines work and other medicines may affect the way doxepin hydrochloride capsules works.
Especially tell your health care provider if you take:
- medicines used to treat mood, anxiety, psychotic, or thought disorders, including selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs)
- medicines to treat migraine headaches known as triptans
- other tricyclic antidepressants
- tetracyclic antidepressants
- opioids
- lithium
- tryptophan
- buspirone
- St. John’s Wort
- carbamazepine
- cimetidine
- tolazamide
- medicines that can cause drowsiness
Do not start or stop any other medicines during treatment with doxepin hydrochloride capsules without first talking to your healthcare provider.
Know the medicines you take. Keep a list of them to show to your healthcare providers when you start to take a new medicine.
How should I take doxepin hydrochloride capsules?
- Take doxepin hydrochloride capsules exactly as your health care provider tells you to take it. Do not change your dose or stop taking doxepin hydrochloride capsules without first talking to your healthcare provider.
- Your health care provider may need to change the dose of doxepin hydrochloride capsules until it is the right dose for you.
- If you miss a dose of doxepin hydrochloride capsules, take the missed dose as soon as you remember. If it is almost time for the next dose, do not take the missed dose and take your next dose at the regular time. Do not take two doses of doxepin hydrochloride capsules at the same time.
- If you take too much doxepin hydrochloride capsules, call your healthcare provider or Poison Help Line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What should I avoid while taking doxepin hydrochloride capsules?
- Do not drive a car or another motor vehicle, operate heavy machinery, or do dangerous activities while taking doxepin hydrochloride capsules. Doxepin hydrochloride capsules can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly.
- Do not drink alcohol during treatment with doxepin hydrochloride capsules. Drinking alcohol during treatment with doxepin hydrochloride capsules can increase your risk of having serious side effects.
What are the possible side effects of doxepin hydrochloride capsules?
Doxepin hydrochloride capsules can cause serious side effects, including:
- See
“What is the most important information I should know about doxepin hydrochloride capsules?”
o agitation o seeing or hearing things that are not real (hallucinations) o confusion o coma o fast heartbeat o changes in blood pressure o dizziness o sweating o flushing o high body temperature (hyperthermia) o shaking (tremors), stiff muscles, or muscle twitching o loss of coordination o Seizures o nausea, vomiting, diarrhea -
Eye problems (angle-closure glaucoma).Doxepin hydrochloride capsules may cause a type of eye problem called angle-closure glaucoma in people with certain eye problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. Call your healthcare provider if you have eye pain, changes in your vision, or swelling or redness in or around the eye.
-
Manic episodes.Manic episodes may happen in people with bipolar disorder who take doxepin hydrochloride capsules. Symptoms may include:
o greatly increased energy o severe trouble sleeping o racing thoughts o reckless behaviour o unusually grand ideas o excessive happiness or irritability o talking more or faster than usual -
Seizures (convulsions)
- feeling overly sleepy
- constipation
- dry mouth
- tiredness
- dizziness
These are not all the possible side effects of doxepin hydrochloride capsules.
Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store doxepin hydrochloride capsules?
- Store doxepin hydrochloride capsules at controlled room temperature between 20°C to 25°C (68°F to 77°F).
- Keep doxepin hydrochloride capsules and all medicines out of the reach of children.
General Information about the safe and effective use of doxepin hydrochloride capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take doxepin hydrochloride capsules for a condition for which it was not prescribed. Do not give doxepin hydrochloride capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about doxepin hydrochloride capsules that is written for health professionals.What are the ingredients in doxepin hydrochloride capsules?
Active ingredient:doxepin hydrochloride
Inactive Ingredients: magnesium stearate, pregelatinised starch, silicified microcrystalline cellulose and sodium lauryl sulfate. The hard gelatin capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 10 mg, 25 mg and 50 mg capsule shells contains FD&C Yellow No. 5 and FD&C Red 40; and 75 mg and 100 mg capsules shells contains D&C Yellow 10 and FD&C Green 3.
The imprinting black ink contains black iron oxide, potassium hydroxide and shellac.
For more information, call 1-855-839-8189.
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA, Inc.
Somerset, NJ 08873
Rev. 07/2025This Medication Guide has been approved by the U.S. Food and Drug Administration.
- Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 42571-420-01
Doxepin Hydrochloride Capsules, USP
10 mg*
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
100 Capsules
Micro Labs Limited

NDC: 42571-421-01
Doxepin Hydrochloride Capsules, USP
25 mg*
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
100 Capsules
Micro Labs Limited
NDC: 42571-422-01
Doxepin Hydrochloride Capsules, USP
50 mg*
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
100 Capsules
Micro Labs Limited
NDC: 42571-423-01
Doxepin Hydrochloride Capsules, USP
75 mg*
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
100 Capsules
Micro Labs Limited
NDC: 42571-424-01
Doxepin Hydrochloride Capsules, USP
100 mg*
Pharmacist: Dispense the accompanying
Medication Guide to each patient.
100 Capsules
Micro Labs Limited

-
INGREDIENTS AND APPEARANCE
DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42571-420 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: MB5IUD6JUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (buff opaque) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42571-420-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC: 42571-420-13 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217688 01/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42571-421 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 25 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: MB5IUD6JUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (Ivory opaque cap, White opaque body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42571-421-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC: 42571-421-13 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217688 01/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42571-422 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 50 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: MB5IUD6JUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (Ivory Opaque) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42571-422-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC: 42571-422-13 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217688 01/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42571-423 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 75 mg Inactive Ingredients Ingredient Name Strength POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: MB5IUD6JUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color green (brite lite green opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42571-423-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC: 42571-423-13 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217688 01/01/2024 DOXEPIN HYDROCHLORIDE
doxepin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42571-424 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXEPIN HYDROCHLORIDE (UNII: 3U9A0FE9N5) (DOXEPIN - UNII:5ASJ6HUZ7D) DOXEPIN 100 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: MB5IUD6JUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green (Brite lite green opaque) , white (White Opaque) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42571-424-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC: 42571-424-13 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217688 01/01/2024 Labeler - Micro Labs Limited (862174955) Establishment Name Address ID/FEI Business Operations Micro Labs Limited 915793658 analysis(42571-420, 42571-421, 42571-422, 42571-423, 42571-424) , label(42571-420, 42571-421, 42571-422, 42571-423, 42571-424) , manufacture(42571-420, 42571-421, 42571-422, 42571-423, 42571-424) , pack(42571-420, 42571-421, 42571-422, 42571-423, 42571-424)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.