First Aid Direct Nasal and Sinus Decongestant
First Aid Direct Nasal and Sinus Decongestant by
Drug Labeling and Warnings
First Aid Direct Nasal and Sinus Decongestant by is a Otc medication manufactured, distributed, or labeled by Cintas Corporation, Allegiant Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FIRST AID DIRECT NASAL AND SINUS DECONGESTANT- phenylephrine hcl tablet
Cintas Corporation
----------
First Aid Direct Nasal and Sinus Decongestant
Uses
- temporarily relieves sinus congestion and pressure
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
Adults and children 12 years and over:
- 1 tablet every 4 hours
- do not take more than 6 tablets in 24 hours
Children under 12 years: ask a doctor
Other information
- store at room temperature 20°-25°C (68°-77°F)
- do not use if packet is opened or torn
Inactive ingredients
croscarmellose sodium, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, titanium dioxide
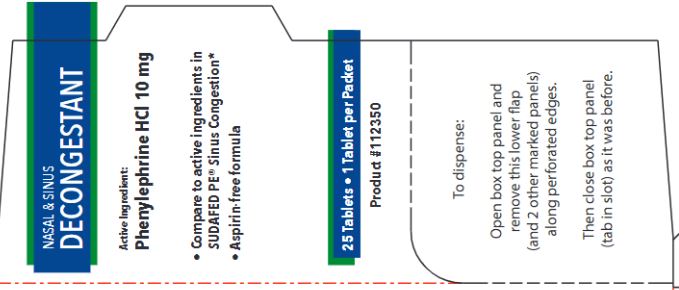
Principal Display Panel - 25 CT Box
NASAL & SINUS DECONGESTANT
- Compare to active ingredients in SUDAFED PE® Sinus Congestion*
- Helps to temporarily relieve: sinus congestion and pressure, nasal congestion due to the common cold, hay fever or other upper respiratory allergies
25 Tablets 1 Tablet per Packet
NASAL & SINUS DECONGESTANT
Active Ingredient:
Phenylephrine HCl 10 mg
- Compare to active ingredients in SUDAFED PE® Sinus Congestion*
- Aspirin-free formula
25 Tablet 1 Tablet per Packet
Product #112350

| FIRST AID DIRECT NASAL AND SINUS DECONGESTANT
phenylephrine hcl tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Cintas Corporation (056481716) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Allegiant Health | 079501930 | manufacture(42961-125) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.