ASPIRIN tablet, delayed release
Aspirin by
Drug Labeling and Warnings
Aspirin by is a Otc medication manufactured, distributed, or labeled by Harris Teeter. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
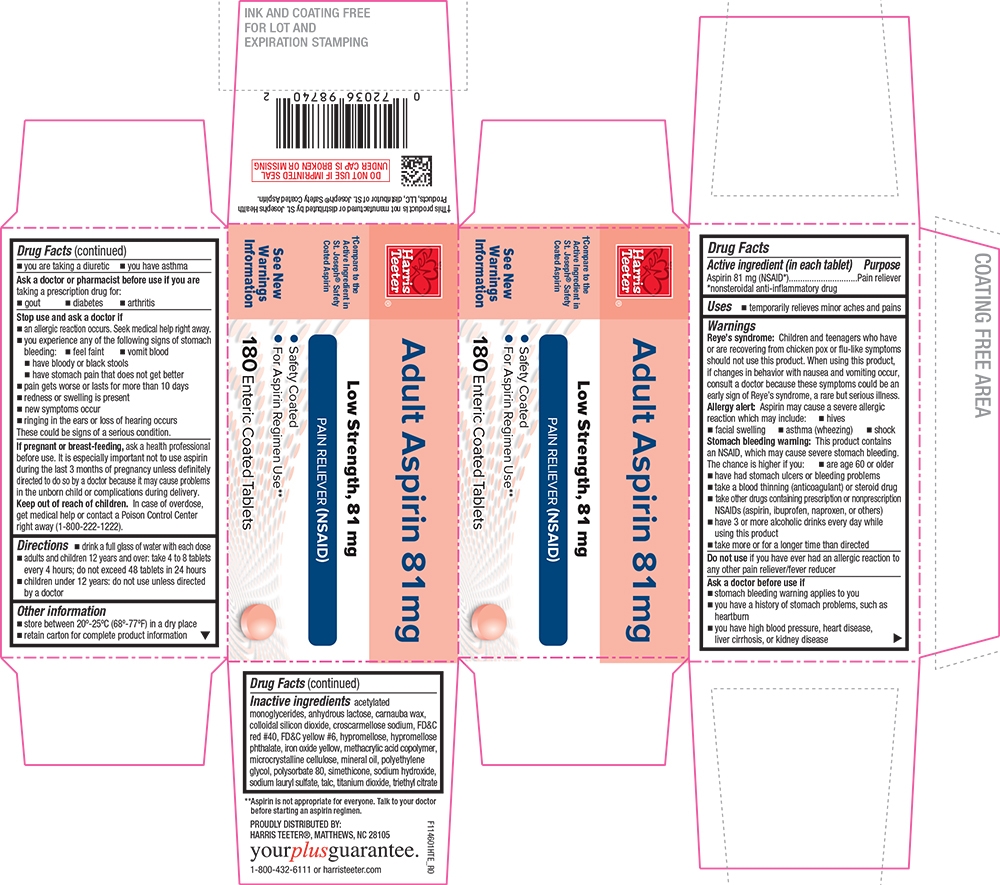
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert
Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are taking a prescription drug for:
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts for more than 10 days
- redness or swelling is present
- new symptoms occur
- ringing in the ears or loss of hearing occurs
These could be signs of a serious condition.
- Directions
- Other information
-
Inactive ingredients
acetylated monoglycerides, anhydrous lactose, carnauba wax, colloidal silicon dioxide, croscarmellose sodium, FD&C red #40, FD&C yellow #6, hypromellose, hypromellose phthalate, iron oxide yellow, methacrylic acid copolymer, microcrystalline cellulose, mineral oil, polyethylene glycol, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPIRIN
aspirin tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72036-146 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength DIACETYLATED MONOGLYCERIDES (UNII: 5Z17386USF) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color orange (PEACH) Score no score Shape ROUND Size 7mm Flavor Imprint Code heart Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72036-146-01 1 in 1 CARTON 10/06/2011 1 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 10/06/2011 Labeler - Harris Teeter (047279351)
Trademark Results [Aspirin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ASPIRIN 76481358 2788617 Dead/Cancelled |
Simon Carter Accessories, Ltd. 2003-01-10 |
 ASPIRIN 75209895 not registered Dead/Abandoned |
Bayer Aktiengesellschaft 1996-12-09 |
 ASPIRIN 73234351 1171777 Dead/Cancelled |
McIntyre; William A. 1979-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.