ENCORE- estradiol, oxytetracycline implant
Encore by
Drug Labeling and Warnings
Encore by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION:
- INDICATIONS:
- USE:
- DIRECTIONS:
- WHEN TO IMPLANT ENCORE:
- WARNING:
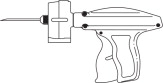
- EQUIPMENT:
-
DIRECTIONS FOR IMPLANTATION:
Insert one implant under the skin of the ear.
- Confine animal in a squeeze chute.
- To reduce the possibility of infection and resulting implant loss, hygienic and antiseptic procedures should be followed during implantation. The ear should be clean and dry. The skin should be cleansed with a suitable antiseptic soap and dried prior to implanting. This is particularly important if the ears are contaminated with urine or feces.
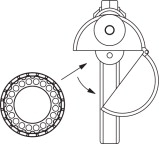
- To load the Compudose/Encore Implanter remove the cartridge from the package, release the latch on the magazine, open and insert the cartridge. (Figure 1) Close the magazine and latch. Advance the cartridge to the next implant by inserting the thumb into the magazine opening and turning the cartridge to the next stop.
Use a sharp needle. The needle should be cleaned and sterilized between each injection by wiping the exterior with a sponge, cloth, or gauze saturated with an appropriate disinfectant. The presence of excessive moisture inside the needle may result in dissolving the oxytetracycline (OTC) from the implant surface and contribute to accumulation of OTC inside the needle.
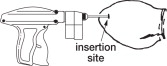
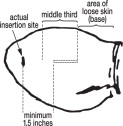
- The implant should be deposited under the skin on the back side of the middle third of the ear. It should be placed between the skin and cartilage, avoiding major blood vessels.
Grasping the tip of the ear with one hand and the implanter in the other, penetrate the skin in the outer third of the ear.
(see Figure 2)
IMPORTANT: Do not penetrate cartilage.
-
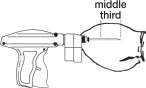
Upon penetration, the needle should be fully inserted (Figure 3) between the skin and cartilage, avoiding major blood vessels. Full insertion of the needle is important to maximize implant retention.
- Pull the needle back as the implant is being deposited by squeezing the lever on the implanter grip. Figure 4 shows the implant in proper position in the middle third of the ear where the skin is tight.
- After repeated use, sufficient oxytetracycline (OTC) from the implant may accumulate inside the needle to impede implant passage. Periodic removal of the needle from the device and washing it in water will prevent such accumulation. Before reusing, the needle should be disinfected and allowed to dry after shaking vigorously to remove excess water or disinfectant.
Proper Position
- If reimplantation is desired, it is not necessary to remove the existing Encore (estradiol implant) implant. Place the second implant at the same level and parallel to, but not in contact with the existing implant or place it in the unimplanted ear in accordance with paragraphs 1-7 of these directions.
CAUTION: To maximize implant retention:
- Fully insert the needle.
- Deposit the implant in the middle third of the ear where the skin is tight.
- Do not deposit the implant where the skin is loose in the third of the ear closest to the head.
The needle has been scientifically designed to maximize retention. When it becomes dull, use a new needle. If the needle is resharpened, sharpen only the point.
Failure to follow antiseptic implanting procedures, particularly when the ears are contaminated with fecal material, may result in infection and excessive implant loss. Implanting cattle during wet weather may increase infection and implant loss.
Carefully check the ears for implant loss approximately 4 weeks after implantation. If loss occurs, reimplant using recommended procedures.
CAUTION: Increased sexual activity (bulling, riding and excitability) has been reported in animals implanted with Encore. Implanted animals should be observed for such signs particularly during the first few days after implanting and animals being excessively ridden (bullers) should be removed to prevent physical injury. Vaginal and rectal prolapse have been reported in heifers implanted with Encore.
CAUTION: Do not use in animals intended for breeding purposes.
- Side Effects:
-
How Supplied:
Encore Implants are supplied in cartridges of 20 implants each.
NADA 118-123, Approved by FDA
Manufactured by a non-sterilizing process.
Encore, Compudose, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
Distributed by Elanco US, Inc.
Greenfield, IN 46140, USAProduct of Germany
To report adverse events, access medical information, or to obtain additional product information, call 1-800-428-4441
- Principal Display Panel - Encore Carton Label
- Principal Display Panel - Encore Cartridge Label
-
INGREDIENTS AND APPEARANCE
ENCORE
estradiol, oxytetracycline implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 58198-0347 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength estradiol (UNII: 4TI98Z838E) (estradiol - UNII:4TI98Z838E) estradiol 43.9 mg oxytetracycline (UNII: X20I9EN955) (oxytetracycline anhydrous - UNII:SLF0D9077S) oxytetracycline 0.5 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-0347-1 5 in 1 CARTON 1 20 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA118123 03/12/1982 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 214580029 API MANUFACTURE
Trademark Results [Encore]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENCORE 98802266 not registered Live/Pending |
Manitowoc Crane Companies, LLC 2024-10-15 |
 ENCORE 98784058 not registered Live/Pending |
Mestek, Inc. 2024-10-03 |
 ENCORE 98695803 not registered Live/Pending |
VERANO IP, LLC 2024-08-13 |
 ENCORE 98581801 not registered Live/Pending |
Brands Infinite (Pty) Ltd 2024-06-03 |
 ENCORE 98561606 not registered Live/Pending |
Encore Memories, Inc. 2024-05-21 |
 ENCORE 98529018 not registered Live/Pending |
Awardco, Inc. 2024-05-01 |
 ENCORE 98490610 not registered Live/Pending |
Odyssey Space Research, LLC 2024-04-09 |
 ENCORE 98486776 not registered Live/Pending |
Balanced Body, Inc. 2024-04-05 |
 ENCORE 98459657 not registered Live/Pending |
Encore Intelligence, Inc. 2024-03-20 |
 ENCORE 98454583 not registered Live/Pending |
Far West Brewing, LLC 2024-03-18 |
 ENCORE 98421369 not registered Live/Pending |
Otava, LLC 2024-02-26 |
 ENCORE 98384788 not registered Live/Pending |
PharmaLink, Inc. 2024-01-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.