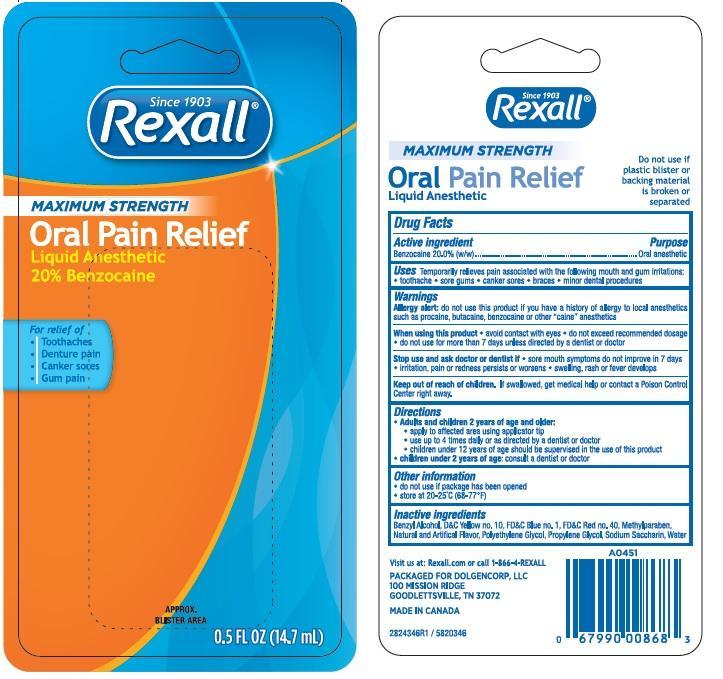
REXALL MAXIMUM STRENGTH- benzocaine liquid
Rexall by
Drug Labeling and Warnings
Rexall by is a Otc medication manufactured, distributed, or labeled by Rexall, Lornamead, CSR Cosmetic Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Uses
-
WARNINGS
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
When using this product
- avoid contact with eyes
- do not exceed recommended dosage
- Do not use more than directed for more than 7 days unless directed by a dentist or doctor.
Stop use and ask a doctor if sore mouth symptoms do not improve in 7 days, swelling, rash or fever develops, irritation, pain or redness persists or worsens swelling rash or fever developes
- Directions
- Other Information
- Inactive ingredients
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REXALL MAXIMUM STRENGTH
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55910-346 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) BENZYL ALCOHOL (UNII: LKG8494WBH) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color orange (dark orange/red to sl brown) Score Shape Size Flavor MINT (Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55910-346-69 1 in 1 CARTON 01/13/2015 1 NDC: 55910-346-61 14.7 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 01/01/2015 Labeler - Rexall (068331990) Registrant - Lornamead (126440440) Establishment Name Address ID/FEI Business Operations CSR Cosmetic Solutions 243501959 manufacture(55910-346) , pack(55910-346)
Trademark Results [Rexall]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REXALL 87087006 not registered Dead/Abandoned |
Safe Harbor Compliance and Clinical Services, LLC 2016-06-28 |
 REXALL 81033322 1033322 Dead/Cancelled |
Rexall Drug Company 0000-00-00 |
 REXALL 78798119 3267813 Dead/Cancelled |
Rexall Sundown, Inc. 2006-01-24 |
 REXALL 73743501 1527754 Live/Registered |
REXALL GROUP, INC., THE 1988-08-01 |
 REXALL 73737721 1524111 Dead/Cancelled |
REXALL GROUP, INC., THE 1988-07-01 |
 REXALL 72465035 0987179 Dead/Expired |
REXALL DRUG COMPANY 1973-08-06 |
 REXALL 72464531 1003147 Dead/Expired |
REXALL DRUG COMPANY 1973-08-02 |
 REXALL 72189209 0784625 Dead/Expired |
REXALL DRUG AND CHEMICAL COMPANY 1964-03-20 |
 REXALL 71084719 0106706 Dead/Expired |
UNITED DRUG COMPANY 1915-02-23 |
 REXALL 71046535 0085696 Dead/Expired |
UNITED DRUG COMPANY 1909-12-15 |
 REXALL 71042358 0090763 Dead/Expired |
UNITED DRUG COMPANY 1909-05-11 |
 REXALL 71038796 0098021 Dead/Expired |
UNITED DRUG COMPANY 1908-11-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.