VAPOR INHALER LEVMETAMFETAMINE NASAL DECONGESTANT, TOPCARE HEALTH- levmetamfetamine inhalant
Vapor Inhaler by
Drug Labeling and Warnings
Vapor Inhaler by is a Otc medication manufactured, distributed, or labeled by Topco Associates LLC, Aphena Pharma Solutions MD, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
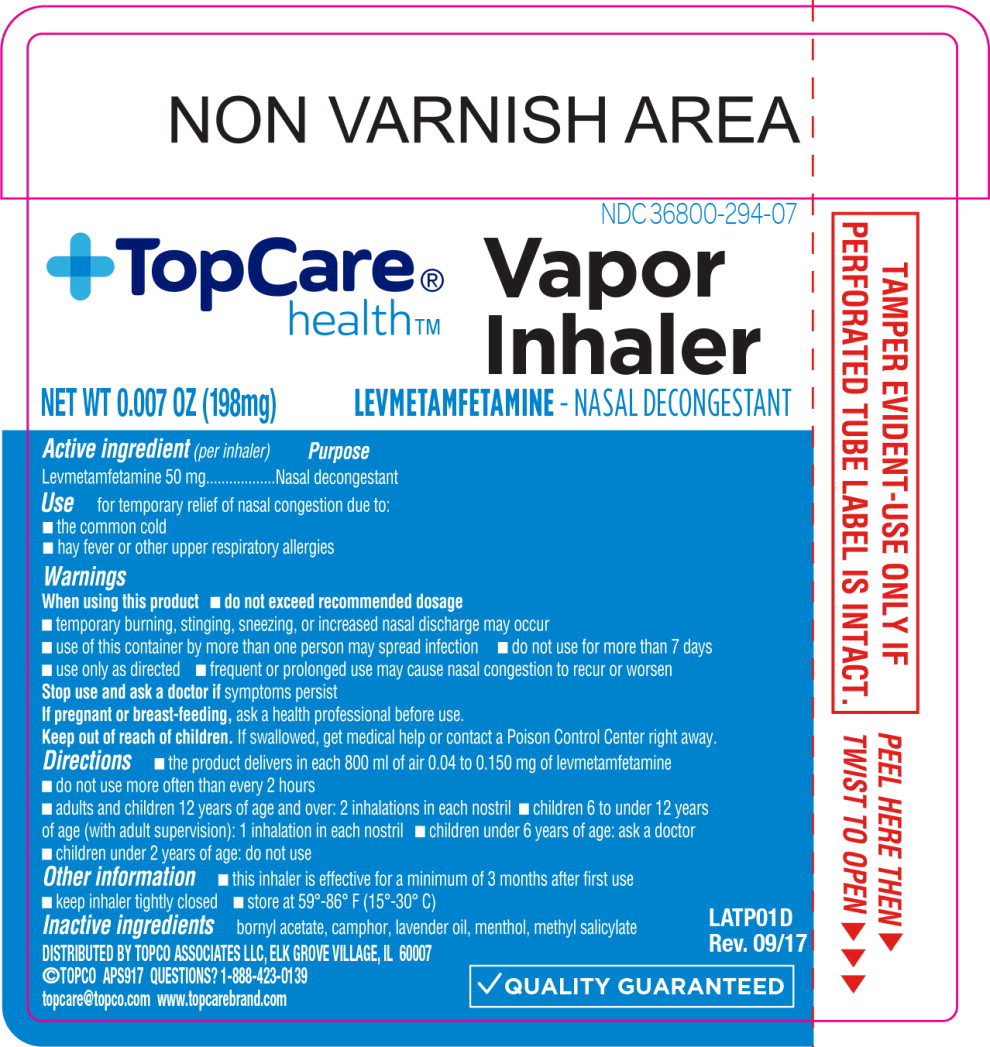
- Active ingredient
- Purpose
- Use:
-
Warnings
When using this product
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
-
Directions
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
adults and children 12 years of age and over 2 inhalations in each nostril children 6 to under 12 years of age (with adult supervision) 1 inhalation in each nostril children under 6 years of age ask a doctor children under 2 years of age do not use - Other information
- Inactive ingredients
-
Principal Display Panel - 198 mg Blister Pack Label
NDC: 36800-294-07
+TopCare® health™
Vapor
InhalerOUR PHARMACISTS
RECOMMENDEDLEVMETAMFETAMINE - NASAL DECONGESTANT
Temporary Relief of Nasal
Congestion Due to:Colds Hay Fever
Upper Respiratory Allergies
NET WT 0.007 OZ (198mg)
-
Principal Display Panel - 198 mg Inhaler Label
NDC: 36800-294-07
+TopCare®
health™Vapor
InhalerLEVMETAMFETAMINE - NASAL DECONGESTANT
NET WT 0.007 OZ (198mg)
-
INGREDIENTS AND APPEARANCE
VAPOR INHALER LEVMETAMFETAMINE NASAL DECONGESTANT, TOPCARE HEALTH
levmetamfetamine inhalantProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36800-294 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levmetamfetamine (UNII: Y24T9BT2Q2) (Levmetamfetamine - UNII:Y24T9BT2Q2) Levmetamfetamine 50 mg Inactive Ingredients Ingredient Name Strength Bornyl acetate (UNII: 213431586X) Camphor (Synthetic) (UNII: 5TJD82A1ET) Lavender oil (UNII: ZBP1YXW0H8) Menthol (UNII: L7T10EIP3A) Methyl Salicylate (UNII: LAV5U5022Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36800-294-07 1 in 1 BLISTER PACK 02/19/2021 1 198 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/19/2021 Labeler - Topco Associates LLC (006935977) Registrant - Aphena Pharma Solutions MD, LLC (829739833) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions MD, LLC 829739833 MANUFACTURE(36800-294)
Trademark Results [Vapor Inhaler]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VAPOR INHALER 74288968 1859297 Dead/Cancelled |
PROCTER & GAMBLE COMPANY, THE 1992-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.