GUAIFENESIN AND CODEINE PHOSPHATE solution
GUAIFENESIN AND CODEINE PHOSPHATE by
Drug Labeling and Warnings
GUAIFENESIN AND CODEINE PHOSPHATE by is a Otc medication manufactured, distributed, or labeled by DirectRX. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION SECTION
-
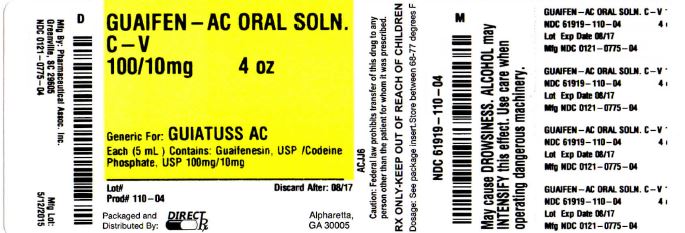
Each 5 mL (1 teaspoonful) contains Guaifenesin, USP 100 mg and Codeine Phosphate, USP 10 mg.
Inactive Ingredients: Citric acid, edetate disodium, FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, flavor, glycerin, menthol, propylene glycol, purified water, sodium benzoate, sodium citrate, sodium saccharin, and sorbitol.
Sodium Content: 5 mg/5 mL
Under federal law, Guaifenesin and Codeine Phosphate Oral Solution USP is available without a prescription. Certain state laws may differ.
-
- CONTRAINDICATIONS SECTION
-
WARNINGS SECTION
-
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash or persistent headache, consult a physician. Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema, or if cough is accompanied by excessive phlegm (mucus) unless directed by a physician. Adults and children who have a chronic pulmonary disease or shortness of breath, or children who are taking other drugs, should not take this product unless directed by a physician. May cause or aggravate constipation. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Professional Note: Guaifenesin has been shown to produce a color interference with certain clinical laboratory determinations of 5-hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
-
- DRUG INTERACTIONS SECTION
-
DOSAGE & ADMINISTRATION SECTION
Take orally as stated below or use as directed by a physician. Adults and children 12 years of age and over: 10 mL (2 teaspoonfuls) every 4 hours, not to exceed 12 teaspoonfuls in a 24-hour period; Children 6 to under 12 years: 5 mL (1 teaspoonful) every 4 hours, not to exceed 6 teaspoonfuls in a 24-hour period; Children under 6 years: consult a physician. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age. Giving a higher dose than recommended by a physician could result in serious side effects for a child. Use of codeine-containing preparations is not recommended for children under 2 years of age. Do not exceed recommended dosage.
- STORAGE AND HANDLING SECTION
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- OTC - KEEP OUT OF REACH OF CHILDREN SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- INDICATIONS & USAGE SECTION
- INACTIVE INGREDIENT SECTION
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND CODEINE PHOSPHATE
guaifenesin and codeine phosphate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61919-110(NDC:0121-0775) Route of Administration oral DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) Guaifenesin 100 mg in 5 mL Codeine Phosphate (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) Codeine Phosphate 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength edetate disodium (UNII: 7FLD91C86K) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Red No. 40 (UNII: WZB9127XOA) FD&C Yellow No. 6 (UNII: H77VEI93A8) menthol (UNII: L7T10EIP3A) propylene glycol (UNII: 6DC9Q167V3) sodium benzoate (UNII: OJ245FE5EU) sodium citrate (UNII: 1Q73Q2JULR) sorbitol (UNII: 506T60A25R) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61919-110-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/01/2015 Labeler - DirectRX (079254320) Establishment Name Address ID/FEI Business Operations DirectRX 079254320 repack(61919-110)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.