CABTREO- clindamycin phosphate/benzoyl peroxide/adapalene gel
Cabtreo by
Drug Labeling and Warnings
Cabtreo by is a Prescription medication manufactured, distributed, or labeled by Bausch Health US LLC, Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CABTREO safely and effectively. See full prescribing information for CABTREO.
CABTREO ®(clindamycin phosphate, adapalene, and benzoyl peroxide) topical gel
Initial U.S. Approval: 2023INDICATIONS AND USAGE
CABTREO is a combination of clindamycin phosphate (a lincosamide antibacterial), adapalene (a retinoid), and benzoyl peroxide indicated for the topical treatment of acne vulgaris in adult and pediatric patients 12 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Topical gel: 1.2% clindamycin phosphate, 0.15% adapalene, and 3.1% benzoyl peroxide. CABTREO is supplied in 20-gram and 50-gram pumps. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity: If a serious hypersensitivity reaction occurs, discontinue CABTREO immediately and initiate appropriate therapy. ( 5.1)
- Colitis: Clindamycin can cause severe colitis, which may result in death. Discontinue CABTREO if diarrhea occurs. ( 5.2)
- Photosensitivity: Avoid or minimize exposure to sunlight and sunlamps. Wear sunscreen and protective clothing when sun exposure cannot be avoided. ( 5.3)
- Skin Irritation and Allergic Contact Dermatitis: Erythema, scaling, dryness, stinging/burning, irritant and allergic contact dermatitis may occur with use of CABTREO and may necessitate discontinuation ( 5.4)
ADVERSE REACTIONS
The most common adverse reactions (occurring in >1% of the CABTREO group and greater than the vehicle group) were application site reactions, pain, erythema, dryness, irritation, exfoliation, and dermatitis. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Neuromuscular Blocking Agents:Use CABTREO with caution in patients receiving neuromuscular blocking agents. ( 7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Colitis
5.3 Photosensitivity
5.4 Skin Irritation and Allergic Contact Dermatitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Cleanse the affected area gently. After the skin is dry, apply a thin layer of CABTREO to the affected area once daily.
Wash hands thoroughly after application of CABTREO.
Avoid the eyes, mouth, paranasal creases, mucous membranes, and areas of broken, eczematous, or sunburned skin [ see Warnings and Precautions ( 5.3)].
CABTREO is for topical use only. Not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria, have been reported with use of clindamycin phosphate, benzoyl peroxide, and adapalene [see Adverse Reactions ( 6.2)]. If a serious hypersensitivity reaction occurs, discontinue CABTREO immediately and initiate appropriate therapy.
5.2 Colitis
Diarrhea, bloody diarrhea, and colitis have been reported with the use of topical and systemic clindamycin. Severe colitis has occurred with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death. Discontinue CABTREO if diarrhea occurs.
5.3 Photosensitivity
CABTREO may increase sensitivity to ultraviolet light. Avoid or minimize sun exposure (including use of tanning beds, and sun lamps) following CABTREO application. Instruct patients to use sunscreen products and wear protective apparel (e.g., hat) when exposure to sun cannot be avoided.
5.4 Skin Irritation and Allergic Contact Dermatitis
Stinging/burning/pain, erythema, dryness, irritation, exfoliation, and dermatitis have been reported with use of CABTREO. These application site adverse reactions occurred at a greater frequency in CABTREO-treated subjects than in vehicle-treated subjects. These adverse reactions are most likely to occur during the first four weeks of treatment [ see Adverse Reactions ( 6.1)] .
Irritant and allergic contact dermatitis have been reported with use of CABTREO.
Weather extremes, such as wind or cold, may be irritating to patients under treatment with CABTREO.
Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of CABTREO, or discontinue use. Avoid applying CABTREO to areas of broken, eczematous, or sunburned skin. Avoid use of “waxing” as a depilatory method on skin treated with CABTREO.
Avoid concomitant use of other potentially irritating topical products such as peeling, desquamating, or abrasive agents and products with high concentrations of alcohol, astringents, spices, or limes.
Use of CABTREO with concomitant topical acne therapy has not been evaluated.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Hypersensitivity [ see Warnings and Precautions ( 5.1)]
- Colitis [see Warnings and Precautions ( 5.2)]
- Skin Irritation and Allergic Contact Dermatitis [see Warnings and Precautions ( 5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In two multicenter, randomized, double-blind, vehicle-controlled clinical trials (Trial 1 and Trial 2), 363 adult and pediatric subjects 10 years of age and older with facial acne vulgaris were treated with CABTREO or vehicle topically once daily for 12 weeks [see Clinical Studies ( 14)] . Adverse reactions reported by >1% of subjects treated with CABTREO and more frequently than subjects treated with vehicle are summarized in Table 1. These adverse reactions were mild (59%), moderate (36.4%), and severe (4.5%). Overall, 2.5% (6/242) of subjects discontinued CABTREO because of local skin reactions.
Table 1: Adverse Reactions Reported by >1% of Subjects with Facial Acne Vulgaris Treated with CABTREO (and More Frequently than Vehicle) in Trials 1 and 2 Adverse Reactions
N (%)CABTREO
N=242Vehicle
N=121- * Application site pain also includes application site stinging and burning
- † Application site erythema also includes erythema
- ‡ Application site dryness also includes xerosis
Application site pain *
33 (13.6)
1 (0.8)
Application site erythema †
11 (4.5)
0
Application site dryness ‡
10 (4.1)
1 (0.8)
Application site irritation
5 (2.1)
0
Application site exfoliation
4 (1.7)
0
Application site dermatitis
3 (1.2)
0
Local tolerability evaluations were conducted at each study visit by assessment of erythema, scaling, itching, burning, and stinging. Table 2 presents the signs and symptoms of local facial tolerability during the 12 Week treatment period in subjects treated with CABTREO.
Table 2: Facial Cutaneous Tolerability Assessment During 12-Week Treatment Period in Subjects with Acne Vulgaris Treated with CABTREO in Trials 1 and 2 - * The denominators for calculating the percentages were the number of subjects with at least one post-baseline cutaneous tolerability assessment.
- † The denominators for calculating the percentages were the number of subjects with Week 12 assessment.
Maximum During Treatment *
Week 12 (End of Treatment) †
Mild
(%)Mod
(%)Severe
(%)Mild
(%)Mod
(%)Severe
(%)CABTREO (N = 242)
Erythema
34.2
19.7
2.1
22.4
6.5
0.5
Burning
29.6
10.7
3.0
4.2
1.4
0.9
Scaling
26.7
3.4
0
7.0
0.9
0
Itching
24.3
3.4
0.4
6.0
0.9
0
Stinging
20.5
5.1
2.6
2.3
0.9
0.5
Vehicle (N = 121)
Erythema
22.5
21.7
1.7
25.5
5.5
0
Burning
2.5
0.8
0.8
0.9
0
0
Scaling
12.5
0
0
4.5
0
0
Itching
11.6
0.8
0
1.8
0
0
Stinging
3.3
0.8
0
1.8
0
0
Local tolerability scores for erythema, scaling, itching, burning, and stinging increased during the first two weeks of treatment and decreased thereafter.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of products containing clindamycin phosphate, adapalene, and benzoyl peroxide as the active ingredients. Because post-marketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders:anaphylaxis and allergic reactions including eyelid edema, throat tightness, swelling of the face, and eczema. [see Contraindications ( 4)] .
Local Adverse Reactions:sunburn, blister, pruritis, hyperpigmentation and hypopigmentation.
Gastrointestinal Disorders:abdominal pain and gastrointestinal disturbances.
Bacterial infections:gram negative folliculitis
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with CABTREO use in pregnant women are insufficient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with CABTREO.
Clindamycin
In published clinical trials and observational studies with pregnant women, oral or IV administration of clindamycin has not been associated with an increased frequency of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, clindamycin phosphate did not cause malformations or embryofetal development toxicity in pregnant rats and mice when administered during the period of organogenesis at systemic doses up to 192 times the maximum recommended human dose (MRHD) of 2.5 g CABTREO, based on a body surface area (mg/m 2) comparison.
Adapalene
Available data from clinical trials with adapalene topical gel use in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of adapalene to pregnant rats and rabbits during organogenesis at doses 64 and 128 times, respectively, the MRHD resulted in fetal skeletal and visceral malformations ( see Data).
Benzoyl peroxide
The systemic exposure of topical benzoyl peroxide is unknown. Based on published literature, benzoyl peroxide is rapidly metabolized to benzoic acid (an endogenous substance), which is eliminated in the urine. Hence, maternal use is not expected to result in fetal exposure of the drug.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Animal reproductive/developmental toxicity studies have not been conducted with CABTREO. Clindamycin phosphate administration during the period of organogenesis in pregnant rats and mice at oral doses up to 600 mg/kg/day (192 and 96 times the MRHD, respectively, based on a mg/m 2comparison) or subcutaneous doses up to 200 mg/kg/day (64 and 32 times the MRHD, respectively, based on a mg/m 2comparison) did not cause fetal malformations or fetotoxicity.
No malformations were observed in rats treated with oral adapalene doses of 0.15 to 5.0 mg/kg/day (up to 13 times the MRHD based on a mg/m 2comparison). However, malformations were observed in rats and rabbits when treated with oral doses of ≥ 25 mg/kg/day adapalene (64 and 128 times the MRHD, respectively, based on a mg/m 2comparison). Findings included cleft palate, microphthalmia, encephalocele, and skeletal abnormalities in rats and umbilical hernia, exophthalmos, and kidney and skeletal abnormalities in rabbits.
Dermal adapalene embryofetal development studies in rats and rabbits at doses up to 6.0 mg/kg/day adapalene (up to 15 and 30 times the MRHD, respectively, based on a mg/m 2comparison) exhibited no fetotoxicity and only minimal increases in skeletal variations (supernumerary ribs in both species and delayed ossification in rabbits).
8.2 Lactation
Risk Summary
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CABTREO and any potential adverse effects on the breastfed child from CABTREO or from the underlying maternal condition.
Clindamycin
There are no data on the presence of clindamycin in human milk, the effects on the breastfed child, or the effects on milk production following topical administration. However, clindamycin has been reported to be present in human milk in small amounts following oral and parenteral administration.
Adapalene
There are no data on the presence of topical adapalene gel or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production. In animal studies, adapalene is present in rat milk with oral administration of the drug. When a drug is present in animal milk, it is likely that the drug will be present in human milk. It is possible that topical administration of large amounts of adapalene could result in sufficient systemic absorption to produce detectable quantities in human milk ( see Clinical Considerations).
Benzoyl peroxide
There are no data on the presence of topical benzoyl peroxide in human milk, its effects on the breastfed infant, or its effects on milk production. The systemic exposure of benzoyl peroxide is unknown. Based on the published literature, benzoyl peroxide is rapidly metabolized to benzoic acid (an endogenous substance), which is eliminated in the urine. Any amount of benzoyl peroxide excreted into human milk by a nursing mother would be expected to be rapidly metabolized by tissue and stomach esterases.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breastmilk, use CABTREO on the smallest area of skin and for the shortest duration possible while breastfeeding. To avoid direct infant exposure, advise patients who are breastfeeding not to apply CABTREO directly to the nipple and areola. If applied to the patient’s chest, care should be taken to avoid infant exposure via direct contact with the infant skin.
8.4 Pediatric Use
The safety and effectiveness of CABTREO for the topical treatment of acne vulgaris have been established in pediatric patients 12 years of age and older. Use of CABTREO for this indication is supported by data from two randomized, double-blind, vehicle-controlled trials [ see Clinical Studies ( 14)].
The safety and effectiveness of CABTREO have not been established in pediatric patients younger than 12 years of age.
-
11 DESCRIPTION
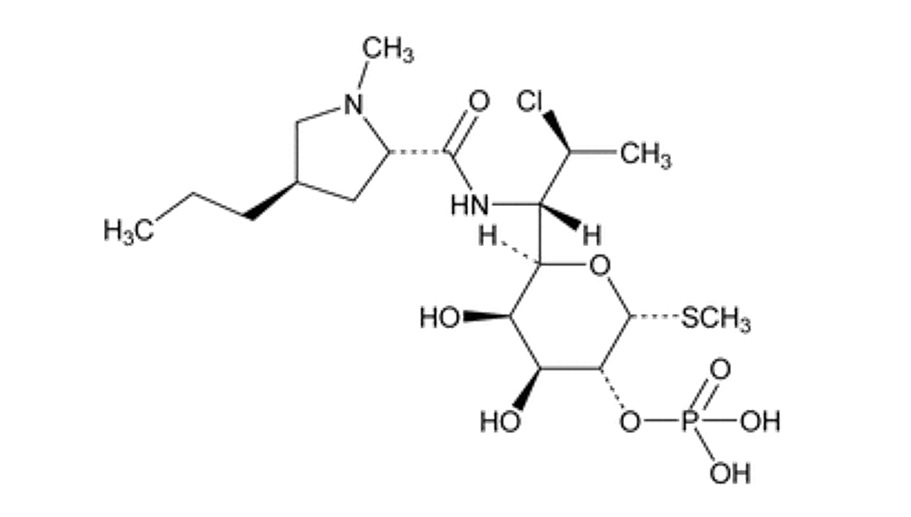
CABTREO (clindamycin phosphate, adapalene, and benzoyl peroxide) topical gel is a white to off-white, opaque gel. Each gram of CABTREO contains 12 mg (1.2%) clindamycin phosphate, equivalent to 10 mg (1%) clindamycin and 1.5 mg (0.15%) adapalene, and 31 mg (3.1%) benzoyl peroxide. Clindamycin phosphate is a water-soluble ester of the semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. Adapalene, a synthetic retinoid, is a naphthoic acid derivative with retinoid-like properties. Benzoyl peroxide is an oxidizing agent.
The chemical name for clindamycin phosphate is Methyl-7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). The structural formula for clindamycin phosphate is represented below:
Clindamycin phosphate:
Molecular Formula: C 18H 34ClN 2O 8PS
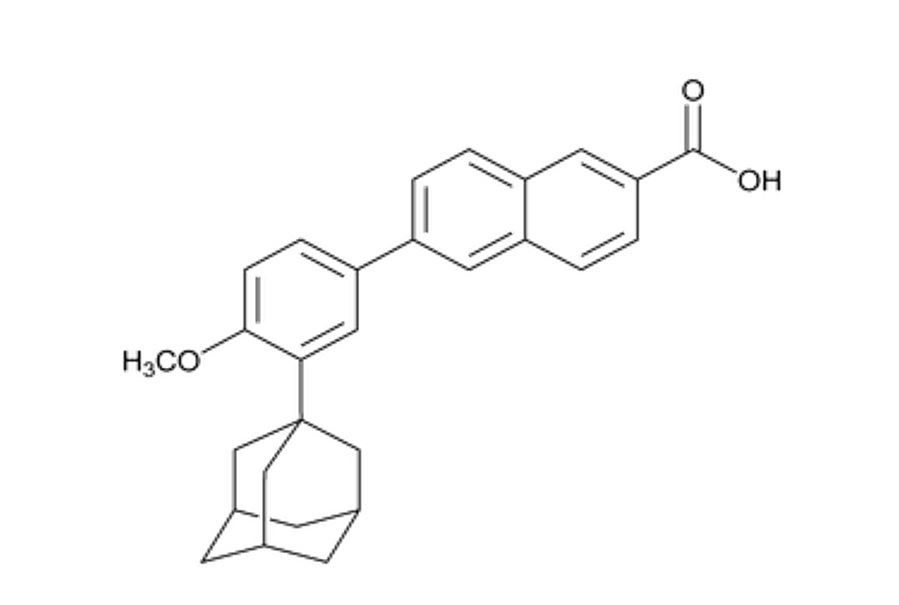
Molecular Weight: 504.97The chemical name for adapalene is 6-[3-(1-Adamantyl)-4-methoxyphenyl]-2-naphthoic acid. The structural formula for adapalene is represented below:
Adapalene:
Molecular Formula: C 28H 28O 3
Molecular Weight: 412.52
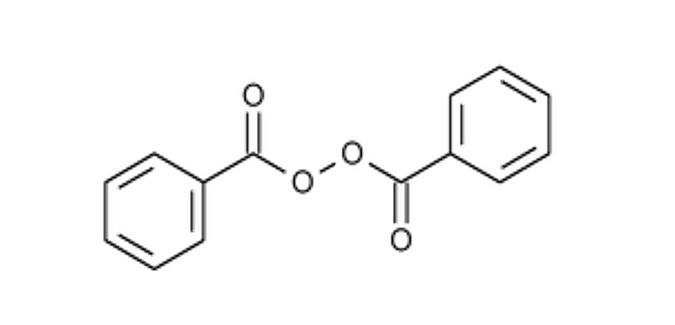
The chemical name for benzoyl peroxide is dibenzoyl peroxide. The structural formula for benzoyl peroxide is represented below:
Benzoyl peroxide:
Molecular Formula: C 14H 10O 4
Molecular Weight: 242.23CABTREO contains the following inactive ingredients: carbomer homopolymer type C (carbomer 980), potassium hydroxide, propylene glycol, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clindamycin:Clindamycin is a lincosamide antibacterial [see Microbiology ( 12.4)] .
Adapalene:Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization and inflammatory processes. However, the significance of these findings with regard to the mechanism of action of adapalene for the treatment of acne is unknown.
Benzoyl Peroxide:Benzoyl peroxide is an oxidizing agent with bactericidal and keratolytic effects but the precise mechanism of action is unknown.
12.3 Pharmacokinetics
Systemic exposure following topical application of CABTREO was evaluated in 28 subjects in an open-label, randomized, pharmacokinetic study. Subjects aged 12 years and older with moderate to severe acne vulgaris applied 2.2 ± 0.4 (mean ± SD) grams of CABTREO to the entire face (excluding eyes and lips), neck, upper chest, upper back and shoulders once daily for 28 days.
Clindamycin phosphate concentrations were measurable in the majority of samples following single and repeated topical administration of CABTREO (LOQ = 0.05 ng/mL). The mean C maxand mean AUC [0-t]values for clindamycin phosphate were 2.44 ng/mL (range: 0.15 to 6.14 ng/mL) and 30.7 ngh/mL (range: 1.04 to 87.4 ngh/mL) on Days 28-29, respectively. Clindamycin (C maxand AUC [0-t]) accumulated up to approximately 3-fold between Days 1-2 and Days 28-29 following once daily application of CABTREO.
Adapalene concentrations were measurable in the majority of samples following single and repeated topical administration of CABTREO (LOQ = 0.10 ng/mL). The mean C maxand mean AUC (0-t)values for adapalene were 0.10 ng/mL (range: 0 to 0.27 ng/mL) and 2.40 ngh/mL (range: 0.56 to 3.87 ngh/mL) on Days 28-29, respectively. Adapalene (AUC [0-t]) accumulated up to approximately 3-fold between Days 1-2 and Days 28-29 for CABTREO.
Benzoyl peroxide is absorbed by the skin where it is converted to benzoic acid and eliminated in the urine.
Drug Interaction Studies
In Vitro Studies
Erythromycin products:Concurrent use with erythromycin topical or oral products may reduce the efficacy of CABTREO. This finding is based upon the mechanistic understanding of these drugs and published in vitro antagonism between erythromycin and clindamycin. This finding was not confirmed by a dedicated clinical study. While the clinical significance of this finding is unknown, it still warrants consideration given the potential impact on the efficacy of CABTREO.
12.4 Microbiology
Clindamycin binds to the 50S ribosomal subunits of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing bacterial protein synthesis.
Clindamycin and benzoyl peroxide individually have been shown to have in vitro activity against Cutibacterium acnes (C. acnes), an organism which has been associated with acne vulgaris. In an in vitro study, the minimum inhibitory concentration (MIC) for benzoyl peroxide against C. acnesis 128 mg/L. The clinical significance of this activity against C. acnesis not known.
C. acnesresistance to clindamycin has been documented. Resistance to clindamycin is often associated with resistance to erythromycin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, genotoxicity, or fertility studies were conducted with CABTREO.
Carcinogenicity studies have been conducted with a gel formulation containing 1% clindamycin phosphate and 5% benzoyl peroxide. In a 2-year dermal carcinogenicity study in mice, treatment with the gel formulation at doses up to 15,000 mg/kg/day (up to 24 times the MRHD for clindamycin phosphate and up to 47 times the MRHD for benzoyl peroxide based on a mg/m 2comparison) did not cause any increase in tumors. However, in a 2-year dermal carcinogenicity study in rats using a different gel formulation containing 1% clindamycin phosphate and 5% benzoyl peroxide increased the incidence of keratoacanthoma at the treated skin site of male rats treated with 2,000 mg/kg/day (6.4 times the MRHD for clindamycin phosphate and 12.4 times the MRHD for benzoyl peroxide based on a mg/m 2comparison). In an oral (gavage) carcinogenicity study in rats, treatment with the gel formulation at doses up to 3,000 mg/kg/day (up to 10 times the MRHD for clindamycin phosphate and up to 19 times the MRHD for benzoyl peroxide based on a mg/m 2comparison) for up to 97 weeks did not cause any increase in tumors.
Carcinogenicity studies with adapalene were conducted in mice at topical doses of 0.4, 1.3, and 4.0 mg/kg/day of adapalene, and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day of adapalene. The highest dose levels are 5.1 (mice) and 3.8 (rats) times the MRHD based on a mg/m 2comparison. In the rat study, an increased incidence of benign and malignant pheochromocytomas reported in the adrenal medulla of male rats was observed.
Benzoyl peroxide is a tumor promoter in several animal species. The significance of this finding in humans is unknown.
Clindamycin phosphate was not genotoxic in the human lymphocyte chromosome aberration assay.
Bacterial mutagenicity assays (Ames test) with benzoyl peroxide provided mixed results; mutagenic potential was observed in a few but not in a majority of investigations. It has been shown to produce single-strand DNA breaks in human bronchial epithelial and mouse epidermal cells, caused DNA-protein cross-links in the human cells, and also induced a dose-dependent increase in sister chromatid exchanges in Chinese hamster ovary cells.
Adapalene did not exhibit mutagenic or genotoxic effects in vitro (Ames test, Chinese hamster ovary cell assay, or mouse lymphoma TK assay) or in vivo (mouse micronucleus test).
Fertility studies in rats treated orally with up to 300 mg/kg/day of clindamycin phosphate (approximately 96 times the MRHD based on a mg/m 2comparison) revealed no effects on fertility or mating ability.
In rat oral studies, 20 mg/kg/day adapalene (51 times the MRHD based on a mg/m 2comparison) did not affect the reproductive performance and fertility of F 0males and females, or the growth, development and reproductive function of F 1offspring.
-
14 CLINICAL STUDIES
The efficacy of CABTREO was evaluated in two multicenter, randomized, double-blind clinical trials (Trial 1 and Trial 2, NCT04214639 and 2 NCT04214652, respectively) in 363 adult and pediatric subjects 10 years of age and older with facial acne vulgaris. While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age.
Subjects were randomized 2:1 to receive CABTREO or vehicle applied once daily for 12 weeks. The trial population included 74% White, 15% Black or African American, 7% Asian, 4% Other, and <1% Native Hawaiian or Other Pacific Islander; for ethnicity, 78% identified as non-Hispanic/Latino and 22% identified as Hispanic/Latino. Fifty-eight percent were female and 42% were male. The median age of the trial population was 18 years (range: 10 to 48 years). Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 non-inflammatory lesions (open and closed comedones) and two or fewer facial nodules. At baseline, most subjects (91%) had EGSS scores that equated to moderate acne. The co-primary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Success on the EGSS was defined as at least a 2-grade improvement from baseline and an EGSS score of clear (0) or almost clear (1).
Table 3 lists the efficacy results for Trials 1 and 2.
Table 3: Efficacy Results at Week 12
Table 3: Efficacy Results at Week 12 in Subjects with Facial Acne Vulgaris in Trials 1 and 2 Trial 1
CABTREO
N=122Vehicle
N=61Treatment Difference (95% Confidence Interval)
EGSS
Clear or Almost Clear and 2-Grade Reduction from Baseline
49.6%
24.9%
24.7 (10.7, 38.7)
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
35.4
23.5
11.9 (7.1, 16.6)
Mean Percent Reduction
72.7%
47.6%
Inflammatory Facial Lesions
Mean Absolute Reduction
27.7
21.7
5.9 (3.1, 8.7)
Mean Percent Reduction
75.7%
59.6%
Trial 2
CABTREO
N=120Vehicle
N=60EGSS
Clear or Almost Clear and
2-Grade Reduction from Baseline
50.5%
20.5%
30.0 (16.4, 43.6)
Non-Inflammatory Facial Lesions
Mean Absolute Reduction
35.2
22.0
13.3 (8.8, 17.7)
Mean Percent Reduction
73.3%
49.0%
Inflammatory Facial Lesions
Mean Absolute Reduction
30.1
20.8
9.3 (6.2, 12.4)
Mean Percent Reduction
80.1%
56.2%
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
CABTREO (clindamycin phosphate, adapalene, and benzoyl peroxide) topical gel is a white to off-white, opaque topical gel. CABTREO contains 1.2% clindamycin phosphate, 0.15% adapalene, and 3.1% benzoyl peroxide and is supplied as follows:
- 20 g pump (NDC: 0187-0006-10)
- 50 g pump (NDC: 0187-0006-25)
Storage and Handling
- Prior to Dispensing:Store CABTREO in a refrigerator between 2° to 8°C (36° to 46°F) until dispensed to the patient. Dispense CABTREO with a 10-week expiration date.
- After Dispensing:Store CABTREO at room temperature at or below 25°C (77°F).
- Do not freeze.
- Keep away from heat.
- Store pump upright.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
Advise patients to apply CABTREO as a thin layer to affected areas, avoiding the eyes, lips, paranasal creases, mucous membranes, and areas of broken, eczematous, or sunburned skin [see Dosage and Administration ( 2)] .
Instruct patients to wash hands after application. [see Dosage and Administration ( 2)] .
Advise patients that CABTREO may bleach hair and colored fabric.
Hypersensitivity
Inform patients that hypersensitivity reactions may occur. Advise patients to discontinue use of CABTREO and contact their healthcare provider immediately if symptoms of an allergic reaction, such as severe swelling or shortness of breath, occur [see Warnings and Precautions ( 5.1), Contraindications ( 4)] .
Colitis
Advise patients to discontinue use of CABTREO and contact their healthcare provider if diarrhea occurs [ see Warnings and Precautions ( 5.2)] .
Photosensitivity
Advise patients to minimize or avoid exposure to sunlight or sunlamps, including tanning beds. Instruct patients to use sunscreen and wear protective clothing (e.g., hat) over treated areas when exposure to sun cannot be avoided [see Warnings and Precautions ( 5.3)] .
Skin Irritation and Allergic Contact Dermatitis
Inform patients that CABTREO may cause irritation, such as erythema, scaling, dryness, itching, stinging or burning. Depending on the severity of the reactions, advise patients to use a moisturizer, reduce the frequency of application, or discontinue use of CABTREO [see Warnings and Precautions ( 5.4)] .
Lactation
Advise patients to use CABTREO on the smallest area of skin and for the shortest duration possible while breastfeeding. To avoid direct infant exposure, instruct patients who are breastfeeding not to apply CABTREO directly to the nipple and areola. Instruct patients to avoid inadvertent contact of treated areas with infant skin [ see Use in Specific Populations ( 8.2)].
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USAManufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, CanadaPatented. See https://patents.ortho-dermatologics.com for US patent information.
CABTREO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
90001201 -
Patient Package Insert
PATIENT INFORMATION
CABTREO ®(kab-TREE-oh)
(clindamycin phosphate, adapalene and benzoyl peroxide)
topical gelImportant information:CABTREO is for use on skin only (topical use). Do not use CABTREO in your mouth, eyes, or vagina.
What is CABTREO?
CABTREO is a prescription medicine used on the skin (topical) to treat acne vulgaris in adults and children 12 years of age and older.
It is not known if CABTREO is safe and effective in children under 12 years of age.
Do not use CABTREO if you have:
- had an allergic reaction to clindamycin, adapalene, benzoyl peroxide, lincomycin, or any of the ingredients in CABTREO. See the end of this Patient Information leaflet for a complete list of ingredients in CABTREO.
- Crohn's disease or ulcerative colitis.
- had inflammation of the colon (colitis), or severe diarrhea with past antibiotic use.
Talk with your healthcare provider if you are not sure if you have any of the conditions listed above.
Before using CABTREO, tell your healthcare provider about all of your medical conditions, including if you:
- plan to have surgery. CABTREO may affect how certain medicines work that may be given during surgery.
- are pregnant or plan to become pregnant. It is not known if CABTREO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if CABTREO passes into your breast milk. Clindamycin when taken by mouth or by injection has been reported to appear in breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with CABTREO.
- If you use CABTREO while breastfeeding:
- use CABTREO on the smallest area of skin and for the shortest time needed.
- do notapply CABTREO directly to the nipple and areola to avoid getting CABTREO into your baby’s mouth.
- avoid direct skin contact with your baby if CABTREO is applied to your chest.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
-
Especially tell your healthcare providerif you take medicine by mouth that contains erythromycin or use products on your skin that contain erythromycin. CABTREO should not be used with products that contain erythromycin.
Especially tell your healthcare provider about any skin products you use. Other skin and topical acne products may increase the irritation of your skin when used with CABTREO.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use CABTREO?
- Use CABTREO exactly as your healthcare provider tells you to use it. See the detailed “Instructions for Use”for directions about how to apply CABTREO.
- Apply a thin layer of CABTREO to cover the treatment area 1 time each day.
- Avoid applying CABTREO to the eyes, lips, creases around your nose, your mouth, and areas of your skin with cuts, abrasions, or sunburned skin.
- Before you apply CABTREO, wash your face gently with a mild or soapless cleanser, rinse with warm water, and pat your skin dry.
- Wash your hands right away after applying CABTREO.
- What should I avoid during treatment with CABTREO?
- Avoid or limit your time in sunlight, including use of tanning beds or sunlamps during treatment with CABTREO. CABTREO can make you more sensitive to the sun, and the light from sunlamps and tanning beds. You could get severe sunburn. Use sunscreen and wear wide-brimmed hat and clothes that cover the treated area of your skin if you have to be in sunlight.
- Cold weather and wind may irritate skin treated with CABTREO.
- Avoid applying CABTREO to areas with skin problems, including cuts, abrasions, sunburn, or eczema.
- Avoid skin products that may dry or irritate your skin such as medicated or harsh soaps, astringents, cosmetics that make your skin dry, and products containing high levels of alcohol, spices, or limes.
- Avoid the use of “waxing” as a hair removal method on skin treated with CABTREO.
- Avoid getting CABTREO in your hair or on colored fabric. CABTREO may bleach hair or colored fabric.
What are the possible side effects of CABTREO?
CABTREO may cause serious side effects, including:
-
Allergic reactions.Stop using CABTREO, and get help right away if you have any of the following symptoms during treatment with CABTREO:
- hives, rash, or severe itching
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing or throat tightness
- feeling faint, dizzy, or lightheaded
- Inflammation of the colon (colitis).Stop using CABTREO and call your healthcare provider right away if you have severe stomach (abdominal) cramps, watery diarrhea, or bloody diarrhea during treatment, and within several weeks after treatment with CABTREO.
- Sensitivity to sunlight.See “What should I avoid while using CABTREO?”
- Skin irritation.Skin irritation is common with CABTREO and is most likely to happen during the first 4 weeks of treatment, and usually lessen with continued use of CABTREO. Skin reactions at the treatment area include redness, scaling, dryness, stinging, burning, itching, and swelling. Tell your healthcare provider if you get any skin reactions.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of CABTREO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CABTREO?
- Store CABTREO at room temperature at or below 77°F (25°C).
- Do not freeze CABTREO.
- Keep CABTREO away from heat.
- Store CABTREO pump upright.
- Throw away (discard) CABTREO that has passed the expiration date.
Keep CABTREO and all medicines out of the reach of children.
General information about the safe and effective use of CABTREO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CABTREO for a condition for which it was not prescribed. Do not give CABTREO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about CABTREO that is written for health professionals.
What are the ingredients in CABTREO?
Active ingredient:clindamycin phosphate, adapalene, and benzoyl peroxide.
Inactive ingredients:carbomer homopolymer type C (carbomer 980), potassium hydroxide, propylene glycol, and purified water.
Distributed by:Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by:Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
Patented. See https://patents.ortho-dermatologics.com for US patent information.
CABTREO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
For more information about CABTREO, call 1-800-321-4576.
- This Patient Information has been approved by the U.S. Food and Drug Administration.
- Revised: 03/2025
- 90001201
-
Instructions for Use
CABTREO ®(kab-TREE-oh)
(clindamycin phosphate, adapalene and benzoyl peroxide)topical gel
This Instructions for Use contains information on how to apply CABTREO.
Important information: CABTREO is for use on skin only (topical use). CABTREO is not for use in your mouth, eyes, or vagina.
Read this Instructions for Use before you start using CABTREO and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
- Apply CABTREO to your face 1 time each day as prescribed.
- Before you apply CABTREO, wash your face gently with a cleanser, rinse with warm water, and pat your skin dry.
Step 1
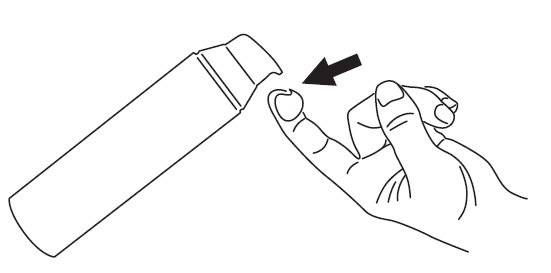
To apply CABTREO to your face, use the pump to dispense one pea-sized amount of CABTREO onto your fingertip. See Figure 1.
- Note: One pea-sized amount of CABTREO should be enough to cover your entire face.
Figure 1
Step 2
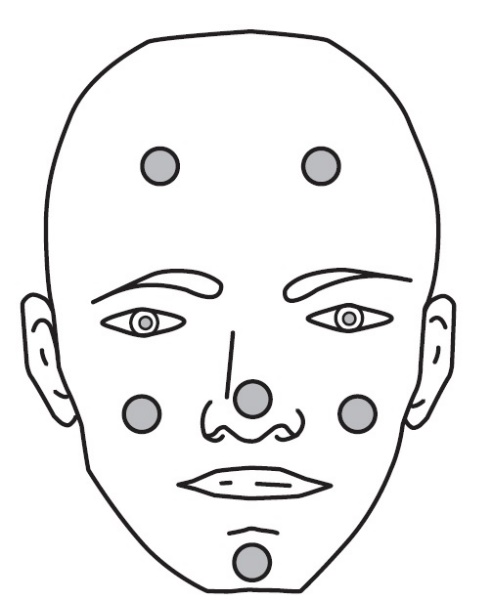
Dot the one pea-sized amount of CABTREO onto six areas of your face (chin, left cheek, right cheek, nose, left forehead, right forehead). See Figure 2.
Figure 2
Step 3
Spread the gel over your face and gently rub it in. It is important to spread the gel over your entire face.If your healthcare provider tells you to put CABTREO on other areas of your skin with acne, be sure to ask how much you should use.
Step 4
Wash your hands after applying CABTREO.
How should I store CABTREO?
- Store CABTREO at room temperature at or below 77°F (25°C).
- Do not freeze CABTREO.
- Keep CABTREO away from heat.
- Store CABTREO pump upright.
- Throw away (discard) CABTREO that has passed the expiration date.
Keep CABTREO and all medicines out of the reach of children.
Distributed by:Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by:Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
Patented. See https://patents.ortho-dermatologics.com for US patent information.
CABTREO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 03/2025
90001201
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CABTREO
clindamycin phosphate/benzoyl peroxide/adapalene gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0187-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 12 mg in 1 g BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 31 mg in 1 g ADAPALENE (UNII: 1L4806J2QF) (ADAPALENE - UNII:1L4806J2QF) ADAPALENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0187-0006-10 1 in 1 CARTON 10/20/2023 1 20 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC: 0187-0006-25 1 in 1 CARTON 10/20/2023 2 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC: 0187-0006-35 3.5 g in 1 TUBE; Type 0: Not a Combination Product 10/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216632 10/20/2023 Labeler - Bausch Health US LLC (831922468) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 245141858 manufacture(0187-0006)
Trademark Results [Cabtreo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CABTREO 97652310 not registered Live/Pending |
Bausch Health Ireland Limited 2022-10-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.