CYSTEX PM- acetaminophen, diphenhydramine hcl tablet
Cystex by
Drug Labeling and Warnings
Cystex by is a Otc medication manufactured, distributed, or labeled by Clarion Brands, LLC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each caplet)
- Purpose
- INDICATIONS & USAGE
-
Warnings
Liver warning:This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- 3 or more alcoholic drinks every day while using this product
- with other drugs containing acetaminophen
Allergy alert:Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
-
Do not use
- with any other product containing diphenhydramine, even one used on skin
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- in children under 12 years of age
- if you have ever had an allergic reaction to this product or any of its ingredients
- Ask a doctor before use if
- Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NEW!
Cystex ® PM
Acetaminophen , Diphenhydramine HCL Pain Reliever/Nighttime Sleep Aid
EXTRA STRENGTH PAIN RELIEF
+ SLEEP AID
Extra strength pain relief
+ sleep aid to help fall asleep
ZZZ
Extra strength pain relief
Safe, non-habit forming
10 caplets
actual size
Guaranteed Satisfaction. If you are not completely satisfied, send us a copy of your cash register receipt, UPC code # along with your full name and address and we will replace or refund your purchase. Limit one refund per household.
USE ONLY IF BLISTER UNIT IS UNBROKEN
Cystex is not intended to replace a doctor's care.
For more information on Cystex tablets or Urinary Tract
Infections please visit www.cystex.com or call 844-297-8394.
Distributed by:
Cystex LLC
27070 Miles Road, Suite A Solon,
OH 44139 4008A ©2022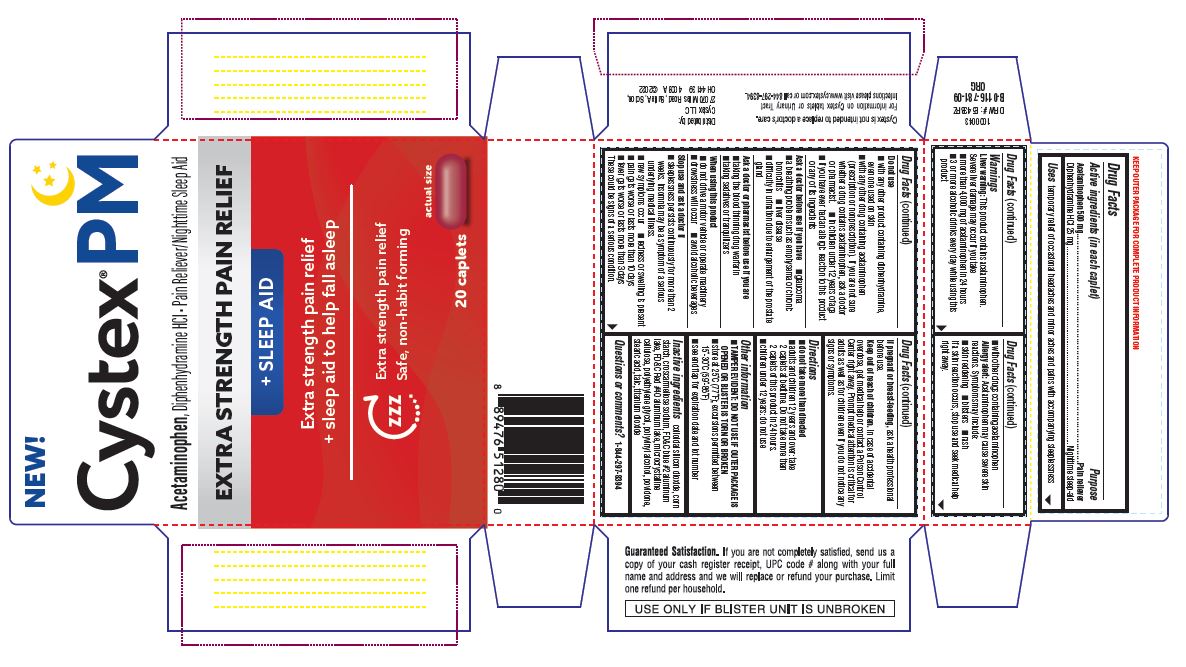
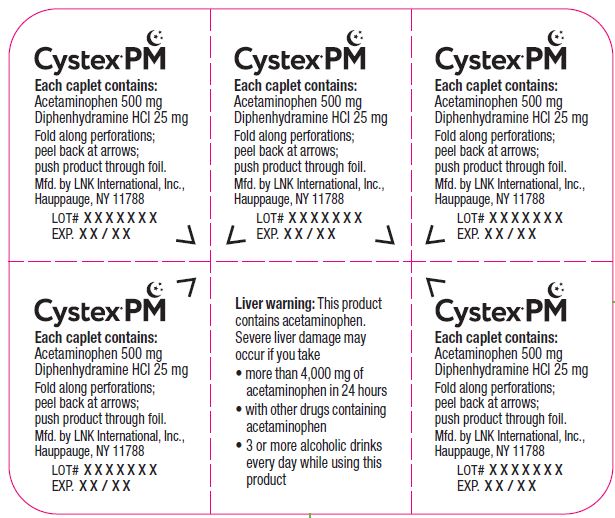
Cystex ® PM
Each caplet contains:
Acetaminophen 500 mg
Diphenhydramine HCL 25 mg
Fold along perforations;
peel back at arrows;
push product through foil.
Distributed by: Cystex LLC
Solon, OH 44139
Lot# XXXXXXX
EXP. XX / XX
Liver warning: This product
contains acetaminophen.
Severe liver damage may
occur if you take
● more than 4,000 mg of acetaminophen in 24 hours
● with other drugs containing acetaminophen
● 3 or more alcoholic drinks every day while using this product
-
INGREDIENTS AND APPEARANCE
CYSTEX PM
acetaminophen, diphenhydramine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69693-417 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C RED NO. 40 (UNII: WZB9127XOA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69693-417-20 1 in 1 CARTON 01/28/2022 1 20 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 69693-417-10 1 in 1 CARTON 06/01/2022 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/28/2022 Labeler - Clarion Brands, LLC (079742703)
Trademark Results [Cystex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYSTEX 88666063 not registered Live/Pending |
Clarion Brands, LLC 2019-10-23 |
 CYSTEX 85489917 4227686 Dead/Cancelled |
DSE Healthcare Solutions, LLC 2011-12-07 |
 CYSTEX 73698088 1514287 Live/Registered |
NUMARK LABORATORIES, INC. 1987-11-30 |
 CYSTEX 73697897 1516189 Dead/Cancelled |
NUMARK LABORATORIES, INC. 1987-11-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.