IFEREX 150- polysaccharide-iron complex capsule
IFEREX 150 by
Drug Labeling and Warnings
IFEREX 150 by is a Other medication manufactured, distributed, or labeled by Nnodum Pharmaceuticals, Contract Pharmacal Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Statement of identity
- Healthn Claims
- Warning
- Dosage and Administration
- Precautions
- Safe Handling Warning
-
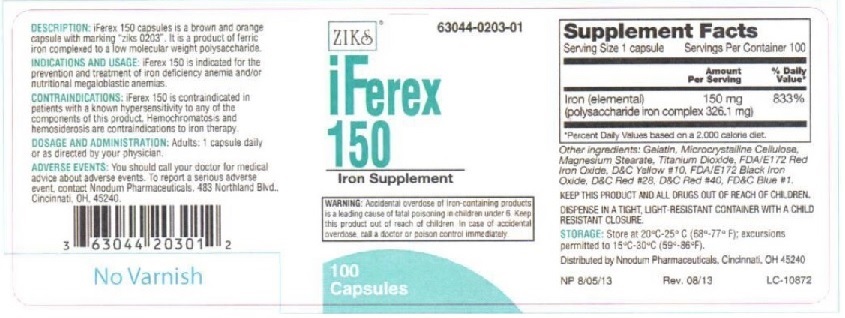
Principal Display Panel - IFEREX 150

ZIKs 63044-0203-01
iFEREX 150
Polysaccharide Iron
Complex Hematinic
100 Capsules
EACH CAPSULE CONTAINS:
Iron (elemental).....................150 mg
(polysaccharide iron complex 326.1 mg)
INACTIVE INGREDIENTS: Black Iron oxide,FD&C Red #28, FD&C Yellow # 10, FD&C blue #1, FD&C red #40, FD&C yellow #6,gelatin, magnesium stearate, microcrystalline cellulose,Red Iron Oxide, and Titanium Dioxide
DESCRIPTION: iFerex 150 is a brown and orange capsule with marking "ziks 0203".
It is a product of ferric iron complexed to a low molecular weight polysaccharide.
INDICATIONS AND USAGE: iFerex 150 is indicated for the prevention and treatment of
iron deficiency anemia and/or nutritional megaloblastic anemias.
CONTRAINDICATIONS: iFerex 150 is indicated in patients with a known hypersensitivity
to any of the components of this product. Hemochromatosis and hemosiderosis are
contraindications to iron therapy.
ADVERSE REACTIONS: Adverse reactions with iron therapy may include constipation,
diarrhea, nausea, vomiting, dark stools, and abdomibal pain. Adverse reactions with iron
tharapy are usually transient.
WARNING: Accidental overdose of iron-containing products is a leading cause in
fatal poisoning of children under 6. Keep this product out of reach of children.
In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS: General: The type of anemia and underlying cause or causes
should be determined before starting therapy with iFerex 150. Since the
anemia may be a result of systemic disturbance, such as recurrent blood loss,
the underlying cause or causes should be corrected, if possible.
DOSAGE AND ADMINISTRATION: Adults: One or two capsules daily or as directed by a physician.
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN.
DISPENSE IN A TIGHT, LIGHT-RESISTANT CONTAINER WITH A RESISTANT CLOSURE.
STORAGE: Store at 20oC-25oC (68o- 77oF); excursions to 15oC-30oC (59o-86oF)
HOW SUPPLIED: iFerex 150 capsules are supplied in 100 capsule per bottle.
Distributed by Nnodum Pharmaceuticals, Cincinnati, OH 45229
NP 6/18/09
LC-10872
3 63044 20301 2
-
INGREDIENTS AND APPEARANCE
IFEREX 150
polysaccharide-iron complex capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:63044-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 150 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:63044-203-61 10 in 1 BOX, UNIT-DOSE 1 10 in 1 BLISTER PACK 2 NHRIC:63044-203-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 03/01/2018 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color color imprint shape size (solid drugs) 7 mm scoring 1 Labeler - Nnodum Pharmaceuticals (960457273) Establishment Name Address ID/FEI Business Operations PD Pharmatech, LLC. 063753431 manufacture
Trademark Results [IFEREX 150]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IFEREX 150 77760548 not registered Dead/Abandoned |
Iferex 150 2009-06-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.