POTASSIUM CITRATE AND CITRIC ACID solution
Potassium Citrate and Citric Acid by
Drug Labeling and Warnings
Potassium Citrate and Citric Acid by is a Prescription medication manufactured, distributed, or labeled by Chartwell RX, LLC, Chartwell Pharmaceuticals Carmel, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Potassium Citrate and Citric Acid Oral Solution, USP is a stable red colored and cherry flavored oral systemic alkalizer containing potassium citrate and citric acid in a sugar-free, non-alcoholic base.
Potassium Citrate and Citric Acid Oral Solution, USP contains in each teaspoonful (5 mL):
Potassium Citrate Monohydrate, USP 1100 mg
Citric Acid Monohydrate, USP 334 mg
Each mL contains 2 mEq potassium ion and is equivalent to 2 mEq bicarbonate (HCO 3).
Inactive Ingredients: saccharin sodium, sodium benzoate, sorbitol solution, FD&C red # 40, purified water, and wild cherry flavor.
ACTIONS
Potassium citrate is absorbed and metabolized to potassium bicarbonate, thus acting as a systemic alkalizer. The effects are essentially those of chlorides before absorption and those of bicarbonates subsequently. Oxidation is virtually complete so that less than 5% of the potassium citrate is excreted in the urine unchanged.
-
INDICATIONS AND USAGE
Potassium citrate and citric acid oral solution is an effective alkalinizing agent useful in those conditions where long-term maintenance of an alkaline urine is desirable, such as in patients with uric acid and cystine calculi of the urinary tract, especially when the administration of sodium salts is undesirable or contraindicated. In addition, it is a valuable adjuvant when administered with uricosuric agents in gout therapy, since urates tend to crystallize out of an acid urine. It is also effective in correcting the acidosis of certain renal tubular disorders where the administration of potassium citrate may be preferable. This product is highly concentrated, and when administered after meals and before bedtime, allows one to maintain an alkaline urinary pH around the clock, usually without the necessity of a 2 A.M. dose. This product alkalinizes the urine without producing a systemic alkalosis in recommended dosage. It is highly palatable, pleasant tasting and tolerable, even when administered for long periods. Potassium citrate does not neutralize the gastric juice or disturb digestion.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Should be used with caution by patients with low urinary output unless under the supervision of a physician. As with all liquids containing a high concentration of potassium, patients should be directed to dilute adequately with water to minimize the possibility of gastrointestinal injury associated with the oral ingestion of concentrated potassium salt preparations; and preferably, to take each dose after meals to avoid saline laxative effect.
-
ADVERSE REACTIONS
Potassium citrate and citric acid oral solution is generally well tolerated without any unpleasant side effects when given in recommended doses to patients with normal renal function and urinary output. However, as with any alkalinizing agent, caution must be used in certain patients with abnormal renal mechanisms to avoid development of hyperkalemia or alkalosis. Potassium intoxication causes listlessness, weakness, mental confusion, tingling of extremities, and other symptoms associated with a high concentration of potassium in the serum. Periodic determinations of serum electrolytes should be carried out in those patients with renal disease in order to avoid these complications. Hyperkalemia may exhibit the following electrocardiographic abnormalities: Disappearance of the P wave, widening and slurring of QRS complex, changes of the S-T segment, tall peaked T waves, etc.
-
OVERDOSAGE
The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are impaired, hyperkalemia can result (see Contraindicationsand Warnings). Hyperkalemia, when detected, must be treated immediately because lethal levels can be reached in a few hours.
TREATMENT OF HYPERKALEMIA
Should hyperkalemia occur, treatment measures include the following: (1) Elimination of foods or medications containing potassium. (2) The intravenous administration of 300 to 500 mL/hr of dextrose solution (10 to 25%), containing 10 units of insulin/20 gm dextrose. (3) The use of exchange resins, hemodialysis, or peritoneal dialysis. In treating hyperkalemia, it should be recalled that in patients who have been stabilized on digitalis, too rapid a lowering of the plasma potassium concentration can produce digitalis toxicity.
-
DOSAGE AND ADMINISTRATION
Potassium Citrate and Citric Acid Oral Solution, USP should be taken diluted in water according to directions, followed by additional water, if desired. Palatability is enhanced if chilled before taking.
Usual Adult Dose
3 to 6 teaspoonfuls (15 to 30 mL), diluted with 1 glass of water, after meals and at bedtime, or as directed by a physician.
Usual Pediatric Dose
1 to 3 teaspoonfuls (5 to 15 mL), diluted with 1/2 glass of water, after meals and at bedtime, or as directed by a physician.
Usual Dosage Range
2 to 3 teaspoonfuls (10 to 15 mL), diluted with a glassful of water, taken four times a day. Potassium Citrate and Citric Acid Oral Solution USP, diluted with a glassful of water, taken four times a day will usually maintain a urinary pH of 7.0-7.6 throughout most of the 24 hours without unpleasant side effects. To check urinary pH, HYDRION Paper (pH 6.0-8.0) or NITRAZINE Paper (pH 4.5-7.5) are available and easy to use.
-
HOW SUPPLIED
Potassium Citrate and Citric Acid Oral Solution, USP (Red color liquid with cherry flavor) is supplied in the following oral dosage form:
16 fl oz (473 mL) bottle NDC: 62135-435-47
5 mL Unit-Dose Cup NDC: 62135-435-05
20 Unit-Dose Cups of 5 mL each NDC: 62135-435-24
STORAGE
Keep tightly closed. Store at controlled room temperature, 20°-25°C (68°-77°F). Protect from excessive heat and freezing.
Manufactured for:
Chartwell RX, LLC
Congers, NY 10920
L71146
Rev. 03/2025 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL
Potassium Citrate and Citric Acid Oral Solution, USP - NDC: 62135-435-47 - 16 fl oz (473 mL) Bottle Label

Potassium Citrate and Citric Acid Oral Solution, USP - NDC: 62135-435- 05 - 5 mL Unit-Dose Cup Label
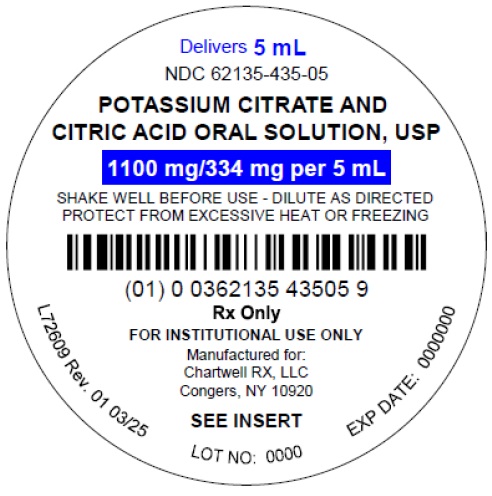
-
INGREDIENTS AND APPEARANCE
POTASSIUM CITRATE AND CITRIC ACID
potassium citrate and citric acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-435 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CITRATE (UNII: EE90ONI6FF) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) POTASSIUM CITRATE 1100 mg in 5 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 334 mg in 5 mL Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL SOLUTION (UNII: 8KW3E207O2) FD&C RED NO. 40 (UNII: WZB9127XOA) WATER (UNII: 059QF0KO0R) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-435-47 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/08/2022 2 NDC: 62135-435-24 2 in 1 BOX 04/04/2025 2 10 in 1 TRAY 2 NDC: 62135-435-05 5 mL in 1 CUP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/08/2022 Labeler - Chartwell RX, LLC (079394054) Registrant - Chartwell Pharmaceuticals Carmel, LLC (118673485) Establishment Name Address ID/FEI Business Operations Chartwell Pharmaceuticals Carmel, LLC 118673485 analysis(62135-435) , manufacture(62135-435) , pack(62135-435)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.