NATRALIA ECZEMA AND PSORIASIS (sodium borate (20x hpus), graphite (6x hpus), potassium sulfate (3x hpus), sodium chloride- 12x hpus shampoo
Natralia Eczema and Psoriasis by
Drug Labeling and Warnings
Natralia Eczema and Psoriasis by is a Homeopathic medication manufactured, distributed, or labeled by Denison Phamaceuticals, LLC, Denison Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
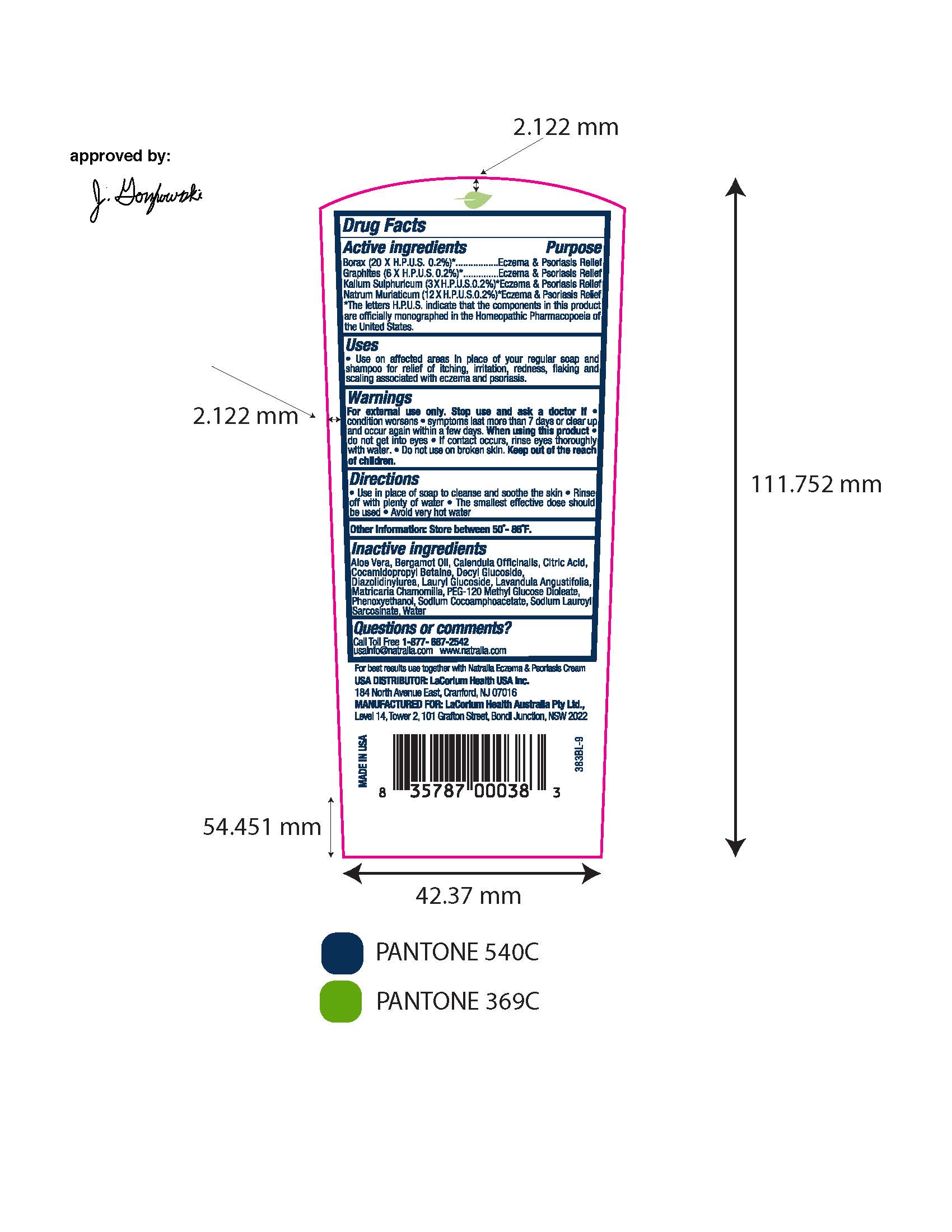
Active ingredients
Borax (20 X H.P.U.S. 0.2%)*
Graphites (6 X H.P.U.S. 0.2%)*
Kalium Sulphuricum (3 X H.P.U.S. 0.2%)*
Natrum Muriaticum (12 X H.P.U.S. 0.2%)*
* The letters H.P.U.S. indicate that the components in this product are officially monographed in the Homeopathic Pharmacopeia of the United States
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only. Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days.
When using this product
- do not get into eyes
- if contact occurs, rinse eyes thoroughly with water
- Do not use on broken skin.
- Keep out of the reach of children
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients
Aloe Vera, Bergamot Oil, Calendula Officinalis, Citric Acid, Cocamidopropyl Betaine, Decyl Glucoside, Diazolidinylurea, Lauryl Glucoside, Lavandula Angustifolia, Matricaria Chamomilla, PEG-120 Methyl Glucose Dioleate, Phenoxyethanol, Sodium Cocoamphoacetate, Sodium Lauroyl Sarcosinate, Water
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATRALIA ECZEMA AND PSORIASIS
sodium borate (20x hpus), graphite (6x hpus), potassium sulfate (3x hpus), sodium chloride (12x hpus) shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0295-9035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 20 [hp_X] in 1 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 6 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 12 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) LAVENDER OIL (UNII: ZBP1YXW0H8) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM COCOAMPHOACETATE (UNII: W7Q5E87674) WATER (UNII: 059QF0KO0R) CHAMOMILE (UNII: FGL3685T2X) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) BERGAMOT OIL (UNII: 39W1PKE3JI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0295-9035-59 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/20/2019 Labeler - Denison Phamaceuticals, LLC (001207208) Establishment Name Address ID/FEI Business Operations Denison Pharmaceuticals, LLC 001207208 manufacture(0295-9035)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.