M-HIST PD- triprolidine hydrochloride liquid
M-Hist PD by
Drug Labeling and Warnings
M-Hist PD by is a Otc medication manufactured, distributed, or labeled by Method Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
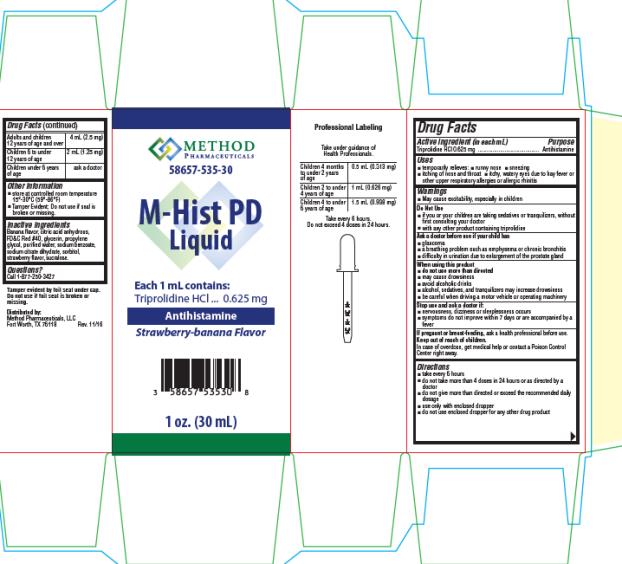
- Active ingredient (in each mL)
- Purpose
- Uses
-
Warnings
- May cause excitability, especially in children
Do Not Use
- if you or your children are taking sedatives or tranquilizers, without first consulting your doctor
- with any other product containing triprolidine
Ask a doctor before use if your child has
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
-
do not use more than directed
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
-
Directions
- take every 6 hours
- do not take more than 4 doses in 24 hours or as directed by a doctor
- do not give more than directed or exceed the recommended daily dosage
- use only with enclosed dropper
- do not use enclosed dropper for any other drug product
Adults and children 12 years of age and over 4 mL (2.5 mg)
Children 6 to under 12 years of age 2 mL (1.25 mg)
Children under 6 years of age ask a doctor- store at controlled room temperature 15°-30°C (59°-86°F)
- Tamper Evident: Do not use if seal is broken or missing.
- take every 6 hours
- Inactive ingredients
-
Questions?
Call 1-877-250-3427
Distributed by:
Method Pharmaceuticals, LLC
Fort Worth, TX 76118Professional Labeling
Take under guidance of Health Professionals.
Children 4 months to under 2 years of age 0.5 mL (0.313 mg)
Children 2 to under 4 years of age 1 mL (0.626 mg)
Children 4 to under 6 years of age 1.5 mL (0.938 mg)Take every 6 hours.
Do not exceed 4 doses in 24 hours.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
M-HIST PD
triprolidine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58657-535 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 0.625 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color red Score Shape Size Flavor BANANA, STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58657-535-30 1 in 1 CARTON 11/08/2016 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 11/08/2016 Labeler - Method Pharmaceuticals, LLC (060216698)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.