TUMS SMOOTHIES- calcium carbonate tablet, chewable
TUMS by
Drug Labeling and Warnings
TUMS by is a Otc medication manufactured, distributed, or labeled by GlaxoSmithKline Consumer Healthcare Holdings (US) LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (per tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
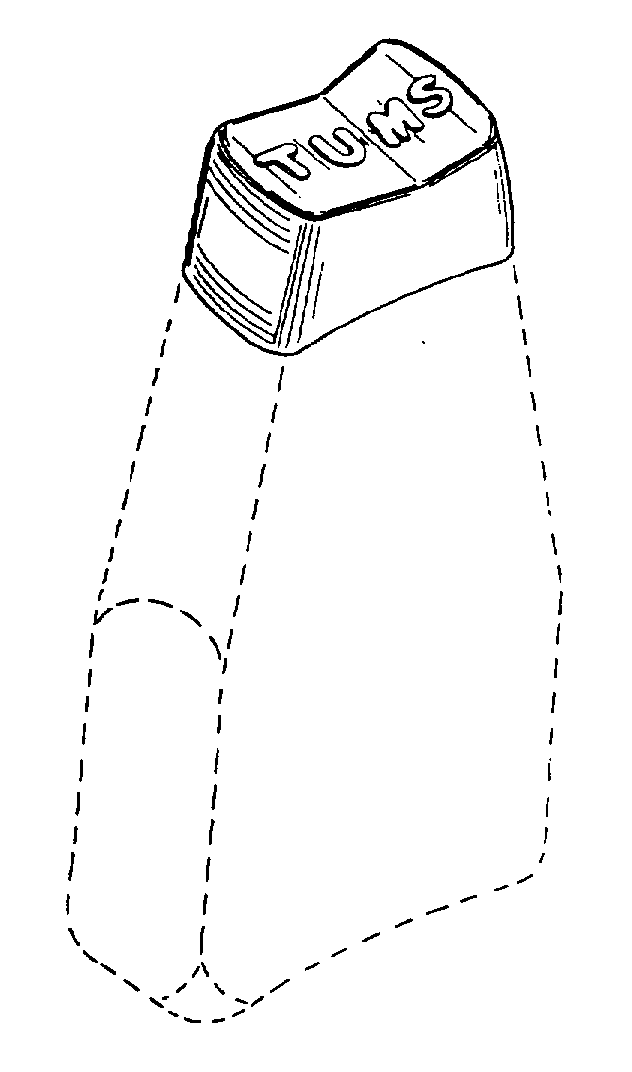
Principal Display Panel
NDC: 0135-0227-01
TUMS
CALCIUM CARBONATE
ANTACID
Smoothies
Strawberry
SMOOTH DISSOLVE
EXTRA STRENGTH 750
GOES TO WORK IN SECONDS!
60CHEWABLE TABLETS
Only Available at DOLLAR GENERAL
PAREVE
Do not use if printed inner safety seal under cap is broken or missing.
Dist. By: GSK CH
Warren, NJ 07059
©2019 GSK or licensor.
Front Panel 1000278
-
INGREDIENTS AND APPEARANCE
TUMS SMOOTHIES
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0135-0227 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) ADIPIC ACID (UNII: 76A0JE0FKJ) CALCIUM STEARATE (UNII: 776XM7047L) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) GUAR GUM (UNII: E89I1637KE) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) Product Characteristics Color PINK (dark pink) Score no score Shape ROUND Size 19mm Flavor STRAWBERRY Imprint Code TUMS;SD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0135-0227-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/07/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 10/07/2019 Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263)
Trademark Results [TUMS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TUMS 98794008 not registered Live/Pending |
Haleon US IP LLC 2024-10-10 |
 TUMS 98058821 not registered Live/Pending |
BANG, Hai Hwa 2023-06-26 |
 TUMS 79373831 not registered Live/Pending |
BANG, Hai Hwa 2023-05-30 |
 TUMS 78226130 not registered Dead/Abandoned |
SmithKline Beecham Corporation 2003-03-17 |
 TUMS 77619345 3741016 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 2008-11-21 |
 TUMS 77155155 not registered Dead/Abandoned |
GLAXOSMITHKLINE LLC 2007-04-12 |
 TUMS 75696786 not registered Dead/Abandoned |
SmithKline Beecham Corporation 1999-05-03 |
 TUMS 75676521 2441507 Dead/Cancelled |
GLAXOSMITHKLINE LLC 1999-04-07 |
 TUMS 75465207 2240777 Dead/Cancelled |
SmithKline Beecham Corporation 1998-04-09 |
 TUMS 75058334 2076469 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) 1996-02-15 |
 TUMS 74723028 1979916 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 1995-08-30 |
 TUMS 74722865 1979915 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 1995-08-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
