MediGummies, COLD & SINUS, Chlorpheniramine 4 mg, Phenylephrine 10mg
MediGummies by
Drug Labeling and Warnings
MediGummies by is a Otc medication manufactured, distributed, or labeled by Harmony Foods D/B/A Santa Cruz Pharmaceuticals, Confab Laboratories Inc., Neopharm Labs Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MEDIGUMMIES ALLERGY AND SINUS- chlorpheniramine maleate and phenylephrine hydrochloride chewable gel
Harmony Foods D/B/A Santa Cruz Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
MediGummies, COLD & SINUS, Chlorpheniramine 4 mg, Phenylephrine 10mg
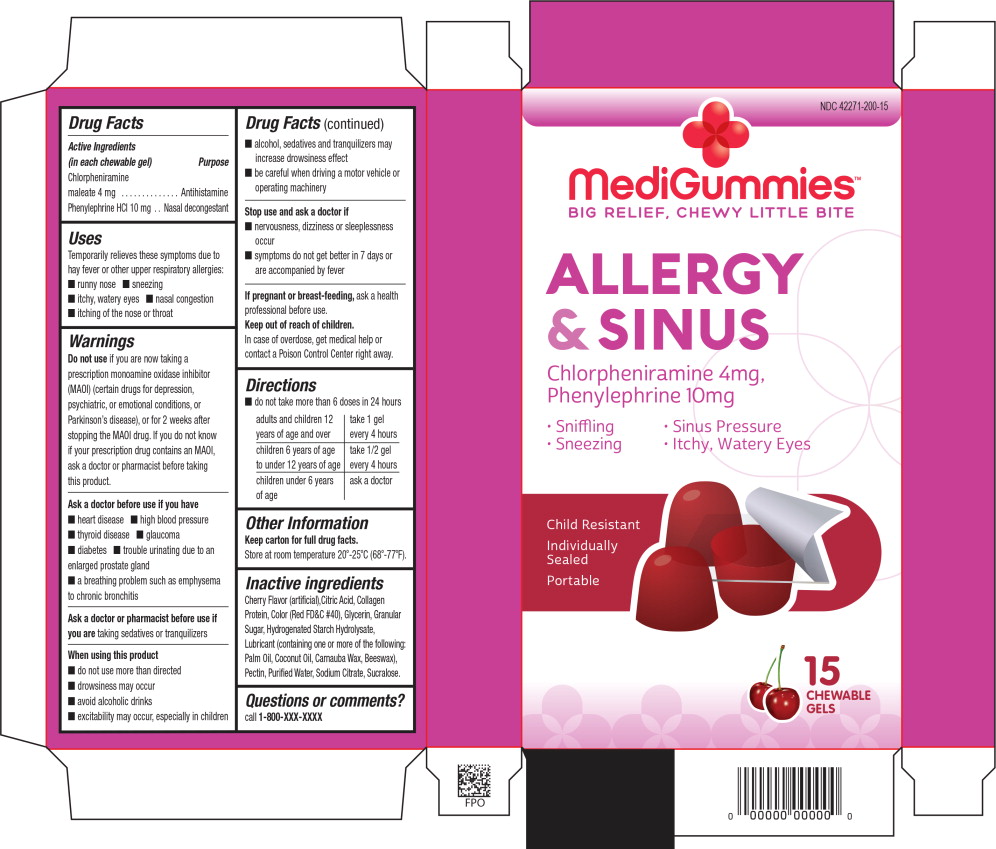
Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- nasal congestion
- itching of the nose or throat
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- glaucoma
- diabetes
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
When using this product
- do not use more than directed
- drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness effect
- be careful when driving a motor vehicle or operating machinery
Directions
- do not take more than 6 doses in 24 hours
| adults and children 12 year of age and over | take 1 gel every 4 hours |
| children 6 years of age to under 12 years of age | take ½ gel every 4 hours |
| children under 6 years of age | ask a doctor |
Inactive ingredients
Cherry Flavor (artificial), Citric Acid, Collagen Protein, Color (Red FD&C #40), Glycerin, Granular Sugar, Hydrogenated Starch Hydrolysate, Lubricant (containing one or more of the following: Palm Oil, Coconut Oil, Carnauba Wax, Beeswax), Pectin, Purified Water, Sodium Citrate, Sucralose.
Principal Display Panel - Allergy and Sinus 15 Carton Label
NDC: 42271-200-15
MediGummies™
BIG RELIEF, CHEWY LITTLE BITE
ALLERGY
& SINUS
Chlorpheniramine 4mg,
Phenylephrine 10mg
Sniffling Sinus Pressure
Sneezing Itchy, Watery Eyes
Child Resistant
Individually
Sealed
Portable
15
CHEWABLE
GELS
Principal Display Panel - Allergy and Sinus 30 Carton Label
NDC: 42271-200-30
MediGummies™
BIG RELIEF, CHEWY LITTLE BITE
ALLERGY
& SINUS
Chlorpheniramine 4mg,
Phenylephrine 10mg
Sniffling Sinus Pressure
Sneezing Itchy, Watery Eyes
Child Resistant
Individually
Sealed
Portable
30
CHEWABLE
GELS
Principal Display Panel - Allergy and Sinus 45 Carton Label
NDC: 42271-200-45
MediGummies™
BIG RELIEF, CHEWY LITTLE BITE
ALLERGY
& SINUS
Chlorpheniramine 4mg,
Phenylephrine 10mg
Sniffling Sinus Pressure
Sneezing Itchy, Watery Eyes
Child Resistant
Individually
Sealed
Portable
45
CHEWABLE
GELS
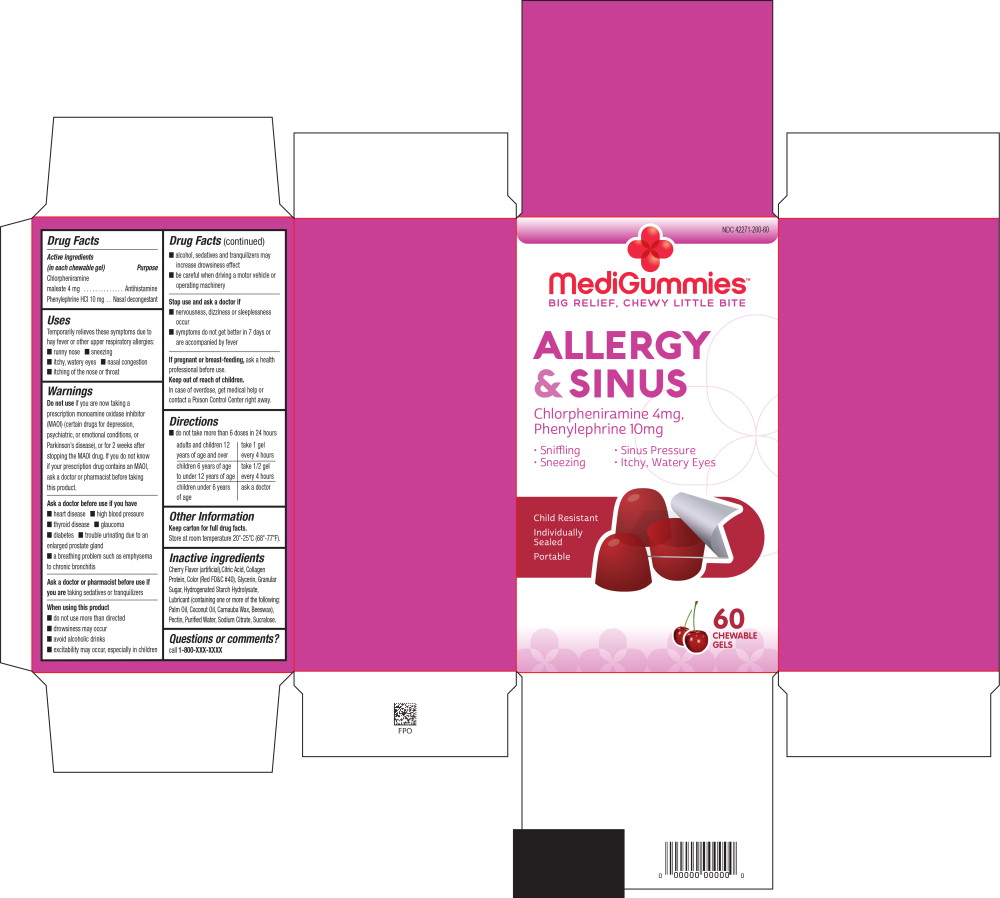
Principal Display Panel - Allergy and Sinus 60 Carton Label
NDC: 42271-200-60
MediGummies™
BIG RELIEF, CHEWY LITTLE BITE
ALLERGY
& SINUS
Chlorpheniramine 4mg,
Phenylephrine 10mg
Sniffling Sinus Pressure
Sneezing Itchy, Watery Eyes
Child Resistant
Individually
Sealed
Portable
60
CHEWABLE
GELS
| MEDIGUMMIES
ALLERGY AND SINUS
chlorpheniramine maleate and phenylephrine hydrochloride chewable gel |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Harmony Foods D/B/A Santa Cruz Pharmaceuticals (093223865) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Confab Laboratories Inc. | 241754217 | manufacture(42271-200) , label(42271-200) , pack(42271-200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neopharm Labs Inc | 243379372 | analysis(42271-200) | |