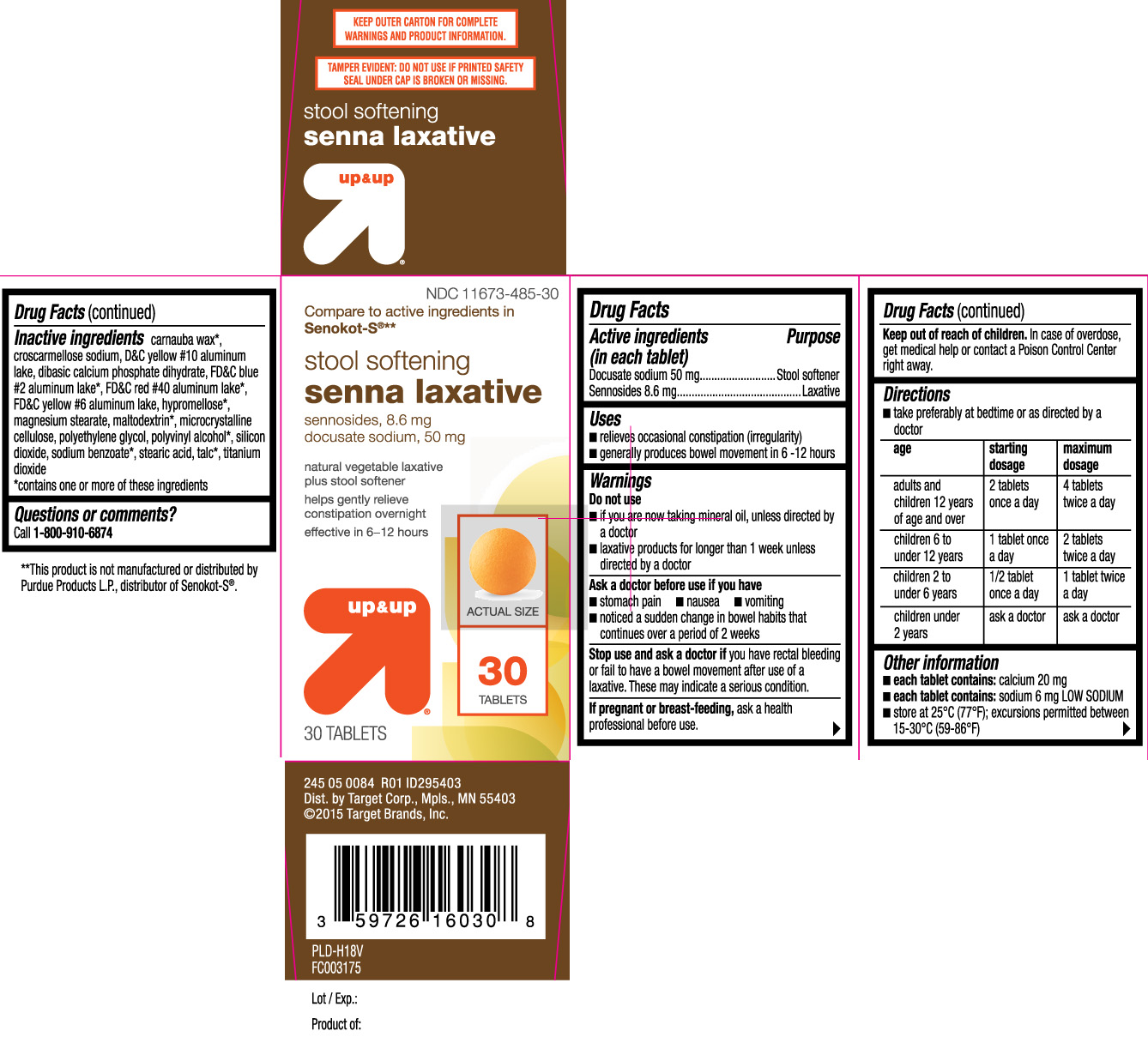
STOOL SOFTENING SENNA LAXATIVE- docusate sodium, sennosides tablet
stool softening by
Drug Labeling and Warnings
stool softening by is a Otc medication manufactured, distributed, or labeled by TARGET Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a periodof 2 weeks
-
Directions
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years of age and over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor
- Other information
-
Inactive ingredients
carnauba wax*, croscarmellose sodium, D&C yellow #10 aluminum lake, dibasic calcium phosphate dihydrate, FD&C blue #2 aluminum lake*, FD&C red #40 aluminum lake*, FD&C yellow #6 aluminum lake, hypromellose*, magnesium stearate, maltodextrin*, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol*, silicon dioxide, sodium benzoate*, stearic acid, talc*, titanium dioxide
*contains one or more of these ingredients
- Questions or comments?
-
Principal Display Panel
Compare to active ingredients in Senokot-S®**
stool softening
senna laxative
sennosides, 8.6 mg
docusate sodium, 50mg
natural vegetable laxative plus stool softener
helps gently relieve constipation overnight
effective in 6-12 hours
TABLETS
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
≠≠This product is not manufactured or distributed by Purdue Products L.P., distributor of Senokot-S®.
Dist. by Target Corp., Mpls., MN 55403
©2015 Target Brand, Inc
- Product Labeling
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENING SENNA LAXATIVE
docusate sodium, sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11673-485 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color ORANGE Score no score Shape ROUND Size 10mm Flavor Imprint Code TCL081;0805;AV;S35 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11673-485-30 1 in 1 BOX 11/30/2015 1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 11/30/2015 Labeler - TARGET Corporation (006961700)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.