Rifampin by American Health Packaging RIFAMPIN capsule
Rifampin by
Drug Labeling and Warnings
Rifampin by is a Prescription medication manufactured, distributed, or labeled by American Health Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Rx only
-
DESCRIPTION
Rifampin capsules, USP for oral administration contain 150 mg or 300 mg rifampin per capsule. The 150 mg and 300 mg capsules contain a powder that is brick-red in color. The 150 mg and 300 mg capsules also contain, as inactive ingredients: colloidal silicon dioxide, D&C Red # 28, FD&C Blue # 1, FD&C Red # 40, gelatin, magnesium stearate, pregelatinized starch, talc, and titanium dioxide.
Rifampin is a semisynthetic antibiotic derivative of rifamycin SV. Rifampin is a red-brown crystalline powder very slightly soluble in water at neutral pH, freely soluble in chloroform, soluble in ethyl acetate and in methanol. Its molecular weight is 822.95 and its chemical formula is C 43H 58N 4O 12. The chemical name for rifampin is either:
3-[[(4-Methyl-1-piperazinyl)imino]methyl]rifamycin
or
5,6,9,17,19,21-hexahydroxy-23-methoxy-2,4,12,16,18,20,22–heptamethyl-8-[N-(4-methyl-1-piperazinyl)formimidoyl]-2,7-(epoxypentadeca [1,11,13] trienimino)naphtho[2,1- b]furan-1,11(2H)-dione 21-acetate.Its structural formula is:
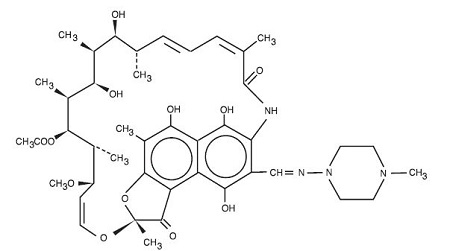
-
CLINICAL PHARMACOLOGY
Oral Administration
Rifampin is readily absorbed from the gastrointestinal tract. Peak serum concentrations in healthy adults and pediatric populations vary widely from individual to individual. Following a single 600 mg oral dose of rifampin in healthy adults, the peak serum concentration averages 7 mcg/mL but may vary from 4 to 32 mcg/mL. Absorption of rifampin is reduced by about 30% when the drug is ingested with food.
Rifampin is widely distributed throughout the body. It is present in effective concentrations in many organs and body fluids, including cerebrospinal fluid. Rifampin is about 80% protein bound. Most of the unbound fraction is not ionized and, therefore, diffuses freely into tissues.
In healthy adults, the mean biological half-life of rifampin in serum averages 3.35 ± 0.66 hours after a 600 mg oral dose, with increases up to 5.08 ± 2.45 hours reported after a 900 mg dose. With repeated administration, the half-life decreases and reaches average values of approximately 2 to 3 hours. The half-life does not differ in patients with renal failure at doses not exceeding 600 mg daily, and consequently, no dosage adjustment is required. The half-life of rifampin at a dose of 720 mg daily has not been established in patients with renal failure. Following a single 900 mg oral dose of rifampin in patients with varying degrees of renal insufficiency, the mean half-life increased from 3.6 hours in healthy adults to 5, 7.3, and 11 hours in patients with glomerular filtration rates of 30 to 50 mL/min, less than 30 mL/min, and in anuric patients, respectively. Refer to the WARNINGS section for information regarding patients with hepatic insufficiency.
After absorption, rifampin is rapidly eliminated in the bile, and an enterohepatic circulation ensues. During this process, rifampin undergoes progressive deacetylation so that nearly all the drug in the bile is in this form in about 6 hours. This metabolite has antibacterial activity. Intestinal reabsorption is reduced by deacetylation, and elimination is facilitated. Up to 30% of a dose is excreted in the urine, with about half of this being unchanged drug.
Pediatrics
Oral Administration
In one study, pediatric patients 6 to 58 months old were given rifampin suspended in simple syrup or as dry powder mixed with applesauce at a dose of 10 mg/kg body weight. Peak serum concentrations of 10.7 ± 3.7 and 11.5 ± 5.1 mcg/mL were obtained 1 hour after preprandial ingestion of the drug suspension and the applesauce mixture, respectively. After the administration of either preparation, the t 1/2 of rifampin averaged 2.9 hours. It should be noted that in other studies in pediatric populations, at doses of 10 mg/kg body weight, mean peak serum concentrations of 3.5 mcg/mL to 15 mcg/mL have been reported.Microbiology
Mechanism of Action
Rifampin inhibits DNA-dependent RNA polymerase activity in susceptible Mycobacterium tuberculosis organisms. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme.Resistance
Organisms resistant to rifampin are likely to be resistant to other rifamycins.In the treatment of both tuberculosis and the meningococcal carrier state (see INDICATIONS AND USAGE), the small number of resistant cells present within large populations of susceptible cells can rapidly become predominant. In addition, resistance to rifampin has been determined to occur as single-step mutations of the DNA-dependent RNA polymerase. Since resistance can emerge rapidly, appropriate susceptibility tests should be performed in the event of persistent positive cultures.
Activity in vitro and in vivo
Rifampin has bactericidal activity in vitro against slow and intermittently growing M tuberculosis organisms.Rifampin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic Gram-Negative Microorganisms:
Neisseria meningitidis“Other” Microorganisms:
Mycobacterium tuberculosisThe following in vitro data are available, but their clinical significance is unknown.
Rifampin exhibits in vitro activity against most strains of the following microorganisms; however, the safety and effectiveness of rifampin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic Gram-Positive Microorganisms:
Staphylococcus aureus (including Methicillin-Resistant S. aureus/MRSA)
Staphylococcus epidermidisAerobic Gram-Negative Microorganisms:
Haemophilus influenzae“Other” Microorganisms:
Mycobacterium lepraeβ-lactamase production should have no effect on rifampin activity.
Susceptibility Testing
For specific information regarding susceptibility test criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: www.fda.gov/STIC.
-
INDICATIONS AND USAGE
In the treatment of both tuberculosis and the meningococcal carrier state, the small number of resistant cells present within large populations of susceptible cells can rapidly become the predominant type. Bacteriologic cultures should be obtained before the start of therapy to confirm the susceptibility of the organism to rifampin and they should be repeated throughout therapy to monitor the response to treatment. Since resistance can emerge rapidly, susceptibility tests should be performed in the event of persistent positive cultures during the course of treatment. If test results show resistance to rifampin and the patient is not responding to therapy, the drug regimen should be modified.
Tuberculosis
Rifampin is indicated in the treatment of all forms of tuberculosis.
A three-drug regimen consisting of rifampin, isoniazid, and pyrazinamide (eg, RIFATER ®1) is recommended in the initial phase of short-course therapy which is usually continued for 2 months. The Advisory Council for the Elimination of Tuberculosis, the American Thoracic Society, and Centers for Disease Control and Prevention recommend that either streptomycin or ethambutol be added as a fourth drug in a regimen containing isoniazid (INH), rifampin, and pyrazinamide for initial treatment of tuberculosis unless the likelihood of INH resistance is very low. The need for a fourth drug should be reassessed when the results of susceptibility testing are known. If community rates of INH resistance are currently less than 4%, an initial treatment regimen with less than four drugs may be considered.
Following the initial phase, treatment should be continued with rifampin and isoniazid (eg, RIFAMATE ®2) for at least 4 months. Treatment should be continued for longer if the patient is still sputum or culture positive, if resistant organisms are present, or if the patient is HIV positive.
- 1 RIFATER® (rifampin, isoniazid, and pyrazinamide) is a registered trademark of Sanofi-Aventis U.S.
- 2 RIFAMATE® (rifampin and isoniazid) is a registered trademark of Sanofi-Aventis U.S.
Meningococcal Carriers
Rifampin is indicated for the treatment of asymptomatic carriers of Neisseria meningitidis to eliminate meningococci from the nasopharynx. Rifampin is not indicated for the treatment of meningococcal infection because of the possibility of the rapid emergence of resistant organisms (see WARNINGS).
Rifampin should not be used indiscriminately, and therefore, diagnostic laboratory procedures, including serotyping and susceptibility testing, should be performed for establishment of the carrier state and the correct treatment. So that the usefulness of rifampin in the treatment of asymptomatic meningococcal carriers is preserved, the drug should be used only when the risk of meningococcal disease is high.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of rifampin and other antibacterial drugs, rifampin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
CONTRAINDICATIONS
Rifampin is contraindicated in patients with a history of hypersensitivity to rifampin or any of the components, or to any of the rifamycins (see WARNINGS).
Rifampin is contraindicated in patients who are also receiving ritonavir-boosted saquinavir due to an increased risk of severe hepatocellular toxicity (see PRECAUTIONS, Drug Interactions).
Rifampin is contraindicated in patients who are also receiving atazanavir, darunavir, fosamprenavir, saquinavir, or tipranavir due to the potential of rifampin to substantially decrease plasma concentrations of these antiviral drugs, which may result in loss of antiviral efficacy and/or development of viral resistance.
Rifampin is contraindicated in patients receiving praziquantel since therapeutically effective blood levels of praziquantel may not be achieved. In patients receiving rifampin who need immediate treatment with praziquantel alternative agents should be considered. However, if treatment with praziquantel is necessary, rifampin should be discontinued 4 weeks before administration of praziquantel. Treatment with rifampin can then be restarted one day after completion of praziquantel treatment.
-
WARNINGS
Rifampin has been shown to produce liver dysfunction. Fatalities associated with jaundice have occurred in patients with liver disease and in patients taking rifampin with other hepatotoxic agents. Patients with impaired liver function should be given rifampin only in cases of necessity and then with caution and under strict medical supervision. In these patients, careful monitoring of liver function, especially SGPT/ALT and SGOT/AST should be carried out prior to therapy and then every 2 to 4 weeks during therapy. If signs of hepatocellular damage occur, rifampin should be withdrawn.
In some cases, hyperbilirubinemia resulting from competition between rifampin and bilirubin for excretory pathways of the liver at the cell level can occur in the early days of treatment. An isolated report showing a moderate rise in bilirubin and/or transaminase level is not in itself an indication for interrupting treatment; rather, the decision should be made after repeating the tests, noting trends in the levels, and considering them in conjunction with the patient's clinical condition.
Rifampin has enzyme-inducing properties, including induction of delta amino levulinic acid synthetase. Isolated reports have associated porphyria exacerbation with rifampin administration.
The possibility of rapid emergence of resistant meningococci restricts the use of rifampin to short-term treatment of the asymptomatic carrier state. Rifampin is not to be used for the treatment of meningococcal disease.
Systemic hypersensitivity reactions were reported with rifampin administration. Signs and symptoms of hypersensitivity reactions may include fever, rash, urticaria, angioedema, hypotension, acute bronchospasm, conjunctivitis, thrombocytopenia, neutropenia, elevated liver transaminases or flu-like syndrome (weakness, fatigue, muscle pain, nausea, vomiting, headache, chills, aches, itching, sweats, dizziness, shortness of breath, chest pain, cough, syncope, palpitations). Manifestations of hypersensitivity, such as fever, lymphadenopathy or laboratory abnormalities (including eosinophilia, liver abnormalities) may be present even though rash is not evident. Monitor patients receiving rifampin for signs and/or symptoms of hypersensitivity reactions. If these signs or symptoms occur, discontinue rifampin and administer supportive measures.
Cases of severe cutaneous adverse reactions (SCAR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome have been reported with rifampin. If symptoms or signs of severe cutaneous adverse reactions develop, discontinue rifampin immediately and institute appropriate therapy.
Rifampin may cause vitamin K–dependent coagulation disorders and bleeding (see ADVERSE REACTIONS). Monitor coagulation tests during rifampin treatment (prothrombin time and other coagulation tests) in patients at risk of vitamin K deficiency (such as those with chronic liver disease, poor nutritional status, on prolonged antibacterial drugs or anticoagulants). Consider discontinuation of rifampin if abnormal coagulation tests and/or bleeding occur. Supplemental vitamin K administration should be considered when appropriate.
Postmarketing reports suggest that concomitant administration of high doses of cefazolin and rifampin may prolong the prothrombin time, leading to severe vitamin K–dependent coagulation disorders that may be life-threatening or fatal. Avoid concomitant use of cefazolin and rifampin in patients at increased risk for bleeding. If no alternative treatment options are available, closely monitor prothrombin time and other coagulation tests, and administer vitamin K as indicated.
-
PRECAUTIONS
General
Rifampin should be used with caution in patients with a history of diabetes mellitus, as diabetes management may be more difficult.
Prescribing rifampin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
For the treatment of tuberculosis, rifampin is usually administered on a daily basis. Doses of rifampin greater than 600 mg given once or twice weekly have resulted in a higher incidence of adverse reactions, including the “flu syndrome” (fever, chills and malaise), hematopoietic reactions (leukopenia, thrombocytopenia, or acute hemolytic anemia), cutaneous, gastrointestinal, and hepatic reactions, shortness of breath, shock, anaphylaxis, and renal failure. Recent studies indicate that regimens using twice-weekly doses of rifampin 600 mg plus isoniazid 15 mg/kg are much better tolerated.
Rifampin is not recommended for intermittent therapy; the patient should be cautioned against intentional or accidental interruption of the daily dosage regimen since rare renal hypersensitivity reactions have been reported when therapy was resumed in such cases.
Rifampin has enzyme induction properties that can enhance the metabolism of endogenous substrates including adrenal hormones, thyroid hormones, and vitamin D. Rifampin and isoniazid have been reported to alter vitamin D metabolism. In some cases, reduced levels of circulating 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D have been accompanied by reduced serum calcium and phosphate, and elevated parathyroid hormone.
Information for Patients
Patients should be counseled that antibacterial drugs including rifampin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When rifampin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by rifampin or other antibacterial drugs in the future.
The patient should be told that rifampin may produce a discoloration (yellow, orange, red, brown) of the teeth, urine, sweat, sputum, and tears, and the patient should be forewarned of this. Soft contact lenses may be permanently stained.
Rifampin is a well characterized and potent inducer of drug metabolizing enzymes and transporters and might therefore decrease concomitant drug exposure and efficacy (see DRUG INTERACTIONS). Therefore patients should be advised not to take any other medication without medical advice.
The patient should be advised that the reliability of oral or other systemic hormonal contraceptives may be affected; consideration should be given to using alternative contraceptive measures.
Patients should be instructed to take rifampin either 1 hour before or 2 hours after a meal with a full glass of water.
Patients should be instructed to notify their physician immediately if they experience any of the following: rash, fever, or swollen lymph nodes, loss of appetite, malaise, nausea and vomiting, darkened urine, yellowish discoloration of the skin and eyes, cough, shortness of breath, wheezing, and pain or swelling of the joints.
Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be stressed.
Laboratory Tests
Adults treated for tuberculosis with rifampin should have baseline measurements of hepatic enzymes, bilirubin, serum creatinine, a complete blood count, and a platelet count (or estimate). Baseline tests are unnecessary in pediatric patients unless a complicating condition is known or clinically suspected.
Patients should be seen at least monthly during therapy and should be specifically questioned concerning symptoms associated with adverse reactions. All patients with abnormalities should have follow-up, including laboratory testing, if necessary. Routine laboratory monitoring for toxicity in people with normal baseline measurements is generally not necessary.
Drug Interactions
Pharmacodynamic interactions
Healthy subjects who received rifampin 600 mg once daily concomitantly with saquinavir 1000 mg/ritonavir 100 mg twice daily (ritonavir-boosted saquinavir) developed severe hepatocellular toxicity. Therefore, concomitant use of these medications is contraindicated (see CONTRAINDICATIONS).When rifampin is given concomitantly with other hepatotoxic medications such as halothane or isoniazid, the potential for hepatotoxicity is increased. The concomitant use of rifampin and halothane should be avoided. Patients receiving both rifampin and isoniazid should be monitored close for hepatotoxicity.
Effect of rifampin on other drugs
Induction of Drug Metabolizing Enzymes and Transporters
Drug metabolizing enzymes and transporters affected by rifampin include cytochromes P450 (CYP) 1A2, 2B6, 2C8, 2C9, 2C19, and 3A4, UDP-glucuronyltransferases (UGT), sulfotransferases, carboxylesterases, and transporters including P-glycoprotein (P-gp) and multidrug resistance-associated protein 2 (MRP2). Most drugs are substrates for one or more of these enzyme or transporter pathways and these pathways may be induced by rifampin simultaneously. Therefore, rifampin may accelerate the metabolism and reduce the activity of certain coadministered drugs, and has the potential to perpetuate clinically important drug-drug interactions against many drugs and across many drug classes (Table 1).Table 1 summarizes the effect of rifampin on other drugs or drug classes. Adjust dosages of concomitant drugs based on approved drug labeling and if applicable, therapeutic drug monitoring, unless otherwise specified.
Table 1: Drug Interactions with Rifampin that Affect Concomitant Drug Concentrations * - * Administered with rifampin 600 mg daily, unless otherwise specified
- † Rifampin dosage used concomitantly with the drug(s) is not specified in the proposed package insert.
- ‡ Administered with rifampin 300 mg daily
- § Administered with rifampin 450 mg daily
- ¶ Administered with rifampin 1200 mg daily
- # Rifampin 1200 mg administered as a single oral dose 8 hours before administering a single oral dose of nifedipine 10 mg
- Þ Numerous cases in the literature describe a decrease in glucocorticoid effect when used concomitantly with rifampin. The literature contains reports of acute adrenal crisis or adrenal insufficiency induced by the combination of rifampin-isoniazid-ethambutol or rifampin-isoniazid in patients with Addison’s disease
- ß Administered with rifampin 900 mg daily
- à A tuberculosis treatment regimen including rifampin (600 mg/day) isoniazid (300 mg/day), pyrazinamide (500 mg 3× per day), and pyridoxine (25 mg) was associated with higher than expected doses of nortriptyline were required to obtain a therapeutic drug level. Following the discontinuation of rifampin, the patient became drowsy and the serum nortriptyline levels rose precipitously (3-fold) into the toxic range.
- è Concomitant use with rifampin in 2 children
- ð Administered with rifampin (10 mg/kg daily)
- ø Administered with an antibiotic regimen including rifampin (450 mg/day), isoniazid (300 mg/day), and streptomycin (0.5 g/day) IM
Drug or Drug Class and Prevention or Management
Clinical Effect
Antiretrovirals
Prevention or Management: Concomitant use is contraindicated (See CONTRAINDICATIONS)
Atazanavir
Decrease AUC by 72%
Darunavir †
Substantial decrease in exposure, which may result in loss of therapeutic effect and development of resistance.
Tipranavir
Fosamprenavir ‡
Decrease AUC by 82%
Saquinavir
Decrease AUC by 70%
Coadministration may result in severe hepatocellular toxicity
Antiretrovirals
Prevention or Management: Avoid concomitant use
Zidovudine
Decrease AUC by 47%
Indinavir
Decrease AUC by 92%
Efavirenz
Decrease AUC by 26%
Hepatitis C Antiviral
Prevention or Management: Avoid concomitant use
Daclatasvir
Decrease AUC by 79%
Simeprevir
Decrease AUC by 48%
Sofosbuvir †
Decrease AUC by 72%
Coadministration of sofosbuvir with rifampin, may decrease sofosbuvir plasma concentrations, leading to reduced therapeutic effect of sofosbuvir.
Telaprevir
Decrease AUC by 92%
Systemic Hormonal Contraceptives
Prevention or Management: Advise patients to change to non-hormonal methods of birth control during rifampin therapyEstrogens
Decrease exposure
Progestins
Anticonvulsants
Phenytoin §
Decrease exposured
Antiarrhythmics
Disopyramide
Decrease exposure
Mexiletine
Decrease exposure
Quinidine
Decrease exposure
Propafenone
Decrease AUC by 50%-67%
Tocainide
Decrease exposure
Antiestrogens
Tamoxifen
Decrease AUC by 86%
Toremifene
Decrease steady state concentrations of toremifene in serum
Antipsychotics
Haloperidol
Decrease plasma concentrations by 70%
Oral Anticoagulants
Prevention or Management: Perform prothrombin time daily or as frequently as necessary to establish and maintain the required dose of anticoagulant
Warfarin
Decrease exposure
Antifungals
Fluconazole
Decrease AUC by 23%
Itraconazole
Prevention or Management: Not recommended 2 weeks before and during itraconazole treatmentDecrease exposure
Ketoconazole
Decrease exposure
Beta-blockers
Metoprolol
Decrease exposure
Propranolol
Decrease exposure
Benzodiazepines
Decrease exposure
Benzodiazepine-related drugs
Zopiclone
Decrease AUC by 82%
Zolpidem
Decrease AUC by 73%
Calcium Channel Blockers¶
Diltiazem
Decrease exposure
Nifedipine #
Decrease exposure
Verapamil
Decrease exposure
CorticosteroidsÞ
Prednisolone
Decrease exposure
Cardiac Glycosides
Digoxin
Prevention or Management: Measure serum digoxin concentrations before initiating rifampin. Continue monitoring and increase digoxin dose by approximately 20%-40% as necessary.
Decrease exposure
Digitoxin
Decrease exposure
Fluoroquinolones
Pefloxacin ß
Decrease exposure
Decrease exposure
Oral Hypoglycemic Agents (e.g. sulfonylureas)
Glyburide
Decrease exposure
Rifampin may worsen glucose control of glyburide
Glipizide
Decrease exposure
Immunosuppressive Agents
Cyclosporine
Decrease exposure
Tacrolimus
Prevention or Management: Monitoring of whole blood concentrations and appropriate dosage adjustments of tacrolimus are recommended when rifampin and tacrolimus are used concomitantly.
Decrease AUC by 56%
Narcotic Analgesics
Oxycodone
Decrease AUC by 86%
Morphine
Decrease exposure
Selective 5-HT3 Receptor Antagonists
Ondansetron
Decrease exposure
Statins Metabolized by CYP3A4
Simvastatin
Decrease exposure
Thiazolidinediones
Rosiglitazone
Decrease AUC by 66%
Tricyclic Antidepressants
Nortriptyline à
Decrease exposure
Other Drugs
Enalapril
Decrease active metabolite exposure
Chloramphenicol è
Decrease exposure
Clarithromycin
Decrease exposure
Dapsone
Decrease exposure
Doxycycline ð
Decrease exposure
Irinotecan ø
Prevention or Management: Avoid the use of rifampin, strong CYP3A4 inducer, if possible. Substitute non-enzyme inducing therapies at least 2 weeks prior to initiation of irinotecan therapy
Decrease irinotecan and active metabolite exposure
Levothyroxine
Decrease exposure
Losartan
Parent
Decrease AUC by 30%
Active metabolite (E3174)
Decrease AUC by 40%.
Methadone
In patients well-stabilized on methadone, concomitant administration of rifampin resulted in a marked reduction in serum methadone levels and a concurrent appearance of withdrawal symptoms.
Praziquantel
Prevention or Management: Concomitant use is contraindicated (See CONTRAINDICATIONS)
Decrease plasma praziquantel concentrations to undetectable levels.
Quinine
Prevention or Management: Avoid concomitant use
Decrease AUC by 75%-85%
Telithromycin
Decrease AUC by 86%
Theophylline
Decrease exposure by 20% to 40%
AUC = area under the time-concentration curve
Effect of other drugs on rifampin
Concomitant antacid administration may reduce the absorption of rifampin. Daily doses of rifampin should be given at least 1 hour before the ingestion of antacids. Concomitant use with probenecid and cotrimoxazole increase the concentration of rifampin which may increase the risk of rifampin toxicities. Monitor for adverse reactions associated with rifampin during coadministration.Other Interactions
Atovaquone: Concomitant use of rifampin with atovaquone decrease concentrations of atovaquone and increase concentrations of rifampin which may increase the risk of rifampin toxicities. Coadministration of rifampin with atovaquone is not recommended.Drug/Laboratory Interactions
Cross-reactivity and false-positive urine screening tests for opiates have been reported in patients receiving rifampin when using the KIMS (Kinetic Interaction of Microparticles in Solution) method (e.g., Abuscreen OnLine opiates assay; Roche Diagnostic Systems). Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish rifampin from opiates.
Therapeutic levels of rifampin have been shown to inhibit standard microbiological assays for serum folate and vitamin B 12. Thus, alternate assay methods should be considered. Transient abnormalities in liver function tests (e.g., elevation in serum bilirubin, alkaline phosphatase, and serum transaminases) and reduced biliary excretion of contrast media used for visualization of the gallbladder have also been observed. Therefore, these tests should be performed before the morning dose of rifampin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A few cases of accelerated growth of lung carcinoma have been reported in man, but a causal relationship with the drug has not been established. Hepatomas were increased in female (C3Hf/DP) mice dosed for 60 weeks with rifampin followed by an observation period of 46 weeks, at 20 to 120 mg/kg (equivalent to 0.1 to 0.5 times the maximum dosage used clinically, based on body surface area comparisons). There was no evidence of tumorigenicity in male C3Hf/DP mice or in similar studies in BALB/c mice, or in two year studies in Wistar rats.
There was no evidence of mutagenicity in both prokaryotic ( Salmonella typhi, Escherichia coli) and eukaryotic ( Saccharomyces cerevisiae) bacteria, Drosophila melanogaster, or ICR/Ha Swiss mice. An increase in chromatid breaks was noted when whole blood cell cultures were treated with rifampin. Increased frequency of chromosomal aberrations was observed in vitro in lymphocytes obtained from patients treated with combinations of rifampin, isoniazid, and pyrazinamide and combinations of streptomycin, rifampin, isoniazid, and pyrazinamide.
Pregnancy – Teratogenic Effects
Rifampin has been shown to be teratogenic in rodents. Congenital malformations, primarily spina bifida were increased in the offspring of pregnant rats given rifampin during organogenesis at oral doses of 150 to 250 mg/kg/day (about 1 to 2 times the maximum recommended human dose based on body surface area comparisons). Cleft palate was increased in a dose-dependent fashion in fetuses of pregnant mice treated at oral doses of 50 to 200 mg/kg (about 0.2 to 0.8 times the maximum recommended human dose based on body surface area comparisons). Imperfect osteogenesis and embryotoxicity were also reported in pregnant rabbits given rifampin at oral doses up to 200 mg/kg/day (about 3 times the maximum recommended human dose based on body surface area comparisons). There are no adequate and well-controlled studies of rifampin in pregnant women. Rifampin has been reported to cross the placental barrier and appear in cord blood. Rifampin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy – Non-Teratogenic Effects
When administered during the last few weeks of pregnancy, rifampin can cause postnatal hemorrhages in the mother and infant for which treatment with vitamin K may be indicated.
Nursing Mothers
Because of the potential for tumorigenicity shown for rifampin in animal studies, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Clinical studies of rifampin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Caution should therefore be observed in using rifampin in elderly patients (see WARNINGS).
-
ADVERSE REACTIONS
Gastrointestinal
Heartburn, epigastric distress, anorexia, nausea, vomiting, jaundice, flatulence, cramps, and diarrhea have been noted in some patients. Although Clostridium difficile has been shown in vitro to be sensitive to rifampin, pseudomembranous colitis has been reported with the use of rifampin (and other broad spectrum antibiotics). Therefore, it is important to consider this diagnosis in patients who develop diarrhea in association with antibiotic use. Tooth discoloration (which may be permanent) may occur.
Hepatic
Transient abnormalities in liver function tests (e.g., elevations in serum bilirubin, alkaline phosphatase, serum transaminases) have been observed. Rarely, hepatitis or a shock-like syndrome with hepatic involvement and abnormal liver function tests has been reported.
Hematologic
Thrombocytopenia has occurred primarily with high dose intermittent therapy, but has also been noted after resumption of interrupted treatment. It rarely occurs during well supervised daily therapy. This effect is reversible if the drug is discontinued as soon as purpura occurs. Cerebral hemorrhage and fatalities have been reported when rifampin administration has been continued or resumed after the appearance of purpura.
Rare reports of disseminated intravascular coagulation have been observed.
Leukopenia, hemolytic anemia, decreased hemoglobin, bleeding, and vitamin K–dependent coagulation disorders (abnormal prolongation of prothrombin time or low vitamin K–dependent coagulation factors) have been observed.
Agranulocytosis has been reported very rarely.
Central Nervous System
Headache, fever, drowsiness, fatigue, ataxia, dizziness, inability to concentrate, mental confusion, behavioral changes, muscular weakness, pains in extremities, and generalized numbness have been observed.
Psychoses have been rarely reported.
Rare reports of myopathy have also been observed.
Endocrine
Menstrual disturbances have been observed.
Rare reports of adrenal insufficiency in patients with compromised adrenal function have been observed.
Renal
Elevations in BUN and serum uric acid have been reported. Rarely, hemolysis, hemoglobinuria, hematuria, interstitial nephritis, acute tubular necrosis, renal insufficiency, and acute renal failure have been noted. These are generally considered to be hypersensitivity reactions. They usually occur during intermittent therapy or when treatment is resumed following intentional or accidental interruption of a daily dosage regimen, and are reversible when rifampin is discontinued and appropriate therapy instituted.
Dermatologic
Cutaneous reactions are mild and self-limiting and do not appear to be hypersensitivity reactions. Typically, they consist of flushing and itching with or without a rash. More serious cutaneous reactions which may be due to hypersensitivity occur but are uncommon.
Hypersensitivity Reactions
Occasionally, pruritus, urticaria, rash, pemphigoid reaction, erythema multiforme, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, Drug Reaction with Eosinophilia and Systemic Symptoms syndrome (see WARNINGS), vasculitis, eosinophilia, sore mouth, sore tongue, and conjunctivitis have been observed.
Anaphylaxis has been reported rarely.
Miscellaneous
Edema of the face and extremities has been reported. Other reactions which have occurred with intermittent dosage regimens include "flu syndrome" (such as episodes of fever, chills, headache, dizziness, and bone pain), shortness of breath, wheezing, decrease in blood pressure and shock. The "flu syndrome" may also appear if rifampin is taken irregularly by the patient or if daily administration is resumed after a drug-free interval.
To report SUSPECTED ADVERSE REACTIONS, contact Lannett Company, Inc. at 1-844-834-0530 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Signs and Symptoms
Nausea, vomiting, abdominal pain, pruritus, headache, and increasing lethargy will probably occur within a short time after ingestion; unconsciousness may occur when there is severe hepatic disease. Transient increases in liver enzymes and/or bilirubin may occur. Brownish-red or orange discoloration of the skin, urine, sweat, saliva, tears, and feces will occur, and its intensity is proportional to the amount ingested.
Liver enlargement, possibly with tenderness, can develop within a few hours after severe overdosage; bilirubin levels may increase and jaundice may develop rapidly. Hepatic involvement may be more marked in patients with prior impairment of hepatic function. Other physical findings remain essentially normal. A direct effect upon the hematopoietic system, electrolyte levels, or acid-base balance is unlikely.
Facial or periorbital edema has also been reported in pediatric patients. Hypotension, sinus tachycardia, ventricular arrhythmias, seizures and cardiac arrest were reported in some fatal cases.
Acute Toxicity
The minimum acute lethal or toxic dose is not well established. However, nonfatal acute overdoses in adults have been reported with doses ranging from 9 to 12 gm rifampin. Fatal acute overdoses in adults have been reported with doses ranging from 14 to 60 gm. Alcohol or a history of alcohol abuse was involved in some of the fatal and nonfatal reports. Nonfatal overdoses in pediatric patients ages 1 to 4 years old of 100 mg/kg for one to two doses has been reported.
Treatment
Intensive support measures should be instituted and individual symptoms treated as they arise. The airway should be secured and adequate respiratory exchange established. Since nausea and vomiting are likely to be present, gastric lavage within the first 2 to 3 hours after ingestion is probably preferable to induction of emesis. Following evacuation of the gastric contents, the instillation of activated charcoal slurry into the stomach may help absorb any remaining drug from the gastrointestinal tract. Antiemetic medication may be required to control severe nausea and vomiting.
Active diuresis (with measured intake and output) will help promote excretion of the drug.
For severe cases, extracorporeal hemodialysis may be required. If this is not available, peritoneal dialysis can be used along with forced diuresis.
-
DOSAGE AND ADMINISTRATION
Rifampin can be administered by the oral route (see INDICATIONS AND USAGE).
See CLINICAL PHARMACOLOGY for dosing information in patients with renal failure.
Tuberculosis
Adults: 10 mg/kg, in a single daily administration, not to exceed 600 mg/day, oral
Pediatric Patients: 10-20 mg/kg, not to exceed 600 mg/day, oralIt is recommended that oral rifampin be administered once daily, either 1 hour before or 2 hours after a meal with a full glass of water.
Rifampin is indicated in the treatment of all forms of tuberculosis. A three-drug regimen consisting of rifampin, isoniazid, and pyrazinamide (e.g., RIFATER ®†) is recommended in the initial phase of short-course therapy which is usually continued for 2 months. The Advisory Council for the Elimination of Tuberculosis, the American Thoracic Society, and the Centers for Disease Control and Prevention recommend that either streptomycin or ethambutol be added as a fourth drug in a regimen containing isoniazid (INH), rifampin and pyrazinamide for initial treatment of tuberculosis unless the likelihood of INH resistance is very low. The need for a fourth drug should be reassessed when the results of susceptibility testing are known. If community rates of INH resistance are currently less than 4%, an initial treatment regimen with less than four drugs may be considered.
Following the initial phase, treatment should be continued with rifampin and isoniazid (e.g., RIFAMATE ®‡) for at least 4 months. Treatment should be continued for longer if the patient is still sputum or culture positive, if resistant organisms are present, or if the patient is HIV positive.
Meningococcal Carriers
Adults: For adults, it is recommended that 600 mg rifampin be administered twice daily for two days.
Pediatric Patients: Pediatric patients 1 month of age or older: 10 mg/kg (not to exceed 600 mg per dose) every 12 hours for two days.
Pediatric patients under 1 month of age: 5 mg/kg every 12 hours for two days. -
HOW SUPPLIED
150 mg: Size # 2 maroon/scarlet capsules imprinted with logo "LANNETT" on the cap and "1393" on the body.
Unit dose packages of 30 (3 x 10) NDC: 68084-357-21300 mg: Size # 1 maroon/scarlet capsules imprinted with logo "LANNETT" on the cap and "1315" on the body.
Unit dose packages of 100 (10 x 10) NDC: 68084-358-01Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture and light.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
†RIFATER ® (rifampin, isoniazid, and pyrazinamide) is a registered trademark of Sanofi-Aventis U.S.
‡RIFAMATE ® (rifampin and isoniazid) is a registered trademark of Sanofi-Aventis U.S. -
REFERENCES
- Clinical Laboratory Standards Institute, Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard CLSI Document M24-A, Vol. 23, No. 18, CLSI, Villanova, PA, 2003.
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Eighth Edition. Approved Standard CLSI Document M7-A8, Vol. 29, No. 2, CLSI, Villanova, PA, January 2009.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests – Tenth Edition. Approved Standard CLSI Document M2-A10, Vol. 29, No. 1, CLSI, Villanova, PA, January 2009.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement, CLSI Document M100-S20, Vol. 30, No. 1, CLSI, Villanova, PA, January 2010.
-
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section) contain drug product from Lannett Company, Inc. as follows:
(150 mg / 30 UD) NDC: 68084-357-21 packaged from NDC: 0527-1393
(300 mg / 100 UD) NDC: 68084-358-01 packaged from NDC: 0527-1315Distributed by:
American Health Packaging
Columbus, OH 432178235721/0419
-
Package/Label Display Panel – Carton – 150 mg

NDC 68084- 357-21
Rifampin
Capsules, USP150 mg
30 Capsules (3 x 10) Rx Only
Each Capsule Contains:
Rifampin, USP ................................................................ 150 mgUsual Dosage: See package insert for full prescribing
information.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled Room
Temperature].
Store in a dry place protected from excessive heat.Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 0527-1393, Lannett Company, Inc.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217035721
0235721/0419 - Package/Label Display Panel – Blister – 150 mg Blister
-
Package/Label Display Panel – Carton – 300 mg

NDC 68084- 358-01
Rifampin
Capsules, USP300 mg
100 Capsules (10 x 10) Rx Only
Each Capsule Contains:
Rifampin, USP ............................................................. 300 mgUsual Dosage: See package insert for full prescribing
information.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].
Store in a dry place protected from excessive heat.Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 0527-1315, Lannett Company, Inc.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217035801
0235801/0119 - Package/Label Display Panel – Blister – 300 mg Blister
-
INGREDIENTS AND APPEARANCE
RIFAMPIN
rifampin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68084-357(NDC:0527-1393) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIFAMPIN (UNII: VJT6J7R4TR) (RIFAMPIN - UNII:VJT6J7R4TR) RIFAMPIN 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red (Maroon/Scarlet) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code LANNETT;1393 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68084-357-21 30 in 1 BOX, UNIT-DOSE 10/27/2009 1 NDC: 68084-357-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065390 10/27/2009 RIFAMPIN
rifampin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68084-358(NDC:0527-1315) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIFAMPIN (UNII: VJT6J7R4TR) (RIFAMPIN - UNII:VJT6J7R4TR) RIFAMPIN 300 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red (Maroon/Scarlet) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code LANNETT;1315 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68084-358-01 100 in 1 BOX, UNIT-DOSE 10/27/2009 1 NDC: 68084-358-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065390 10/27/2009 Labeler - American Health Packaging (929561009) Establishment Name Address ID/FEI Business Operations American Health Packaging 929561009 repack(68084-357, 68084-358)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

