WARFARIN SODIUM- warfarin tablet
Warfarin Sodium by
Drug Labeling and Warnings
Warfarin Sodium by is a Prescription medication manufactured, distributed, or labeled by Golden State Medical Supply, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
Warfarin Sodium Tablets
These highlights do not include all the information needed to use WARFARIN SODIUM TABLETS safely and effectively. See full prescribing information for WARFARIN SODIUM TABLETS.
WARFARIN SODIUM tablets, for oral use
Initial U.S. Approval: 1954WARNING: BLEEDING RISK
See full prescribing information for complete boxed warning.
- Warfarin sodium can cause major or fatal bleeding. (5.1)
- Perform regular monitoring of INR in all treated patients. (2.1)
- Drugs, dietary changes, and other factors affect INR levels achieved with warfarin sodium therapy. (7)
- Instruct patients about prevention measures to minimize risk of bleeding and to report signs and symptoms of bleeding. (17)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Warfarin sodium tablets are vitamin K antagonist indicated for:
- Prophylaxis and treatment of venous thrombosis and its extension, pulmonary embolism (1)
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation and/or cardiac valve replacement (1)
- Reduction in the risk of death, recurrent myocardial infarction, and thromboembolic events such as stroke or systemic embolization after myocardial infarction (1)
Limitation of Use
Warfarin sodium tablets, have no direct effect on an established thrombus, nor does it reverse ischemic tissue damage. (1)
DOSAGE AND ADMINISTRATION
- Individualize dosing regimen for each patient, and adjust based on INR response. (2.1, 2.2)
- Knowledge of genotype can inform initial dose selection. (2.3)
- Monitoring: Obtain daily INR determinations upon initiation until stable in the therapeutic range. Obtain subsequent INR determinations every 1 to 4 weeks. (2.4)
- Review conversion instructions from other anticoagulants. (2.8)
DOSAGE FORMS AND STRENGTHS
- Scored tablets: 1, 2, 2.5, 3, 4, 5, 6, 7.5, or 10 mg (3)
CONTRAINDICATIONS
- Pregnancy, except in women with mechanical heart valves ( 4, 5.7, 8.1)
- Hemorrhagic tendencies or blood dyscrasias (4)
- Recent or contemplated surgery of the central nervous system (CNS) or eye, or traumatic surgery resulting in large open surfaces ( 4, 5.8)
- Bleeding tendencies associated with certain conditions (4)
- Threatened abortion, eclampsia, and preeclampsia (4)
- Unsupervised patients with potential high levels of non-compliance (4)
- Spinal puncture and other diagnostic or therapeutic procedures with potential for uncontrollable bleeding (4)
- Hypersensitivity to warfarin or any component of the product (4)
- Major regional or lumbar block anesthesia (4)
- Malignant hypertension (4)
WARNINGS AND PRECAUTIONS
- Tissue necrosis: Necrosis or gangrene of skin or other tissues can occur, with severe cases requiring debridement or amputation. Discontinue warfarin sodium and consider alternative anticoagulants if necessary. (5.2)
- Calciphylaxis: Fatal and serious cases have occurred. Discontinue warfarin sodium and consider alternative anticoagulation therapy. (5.3)
- Acute kidney injury may occur during episodes of excessive anticoagulation and hematuria. (5.4)
- Systemic atheroemboli and cholesterol microemboli: Some cases have progressed to necrosis or death. Discontinue warfarin sodium if such emboli occur. (5.5)
- Heparin-induced thrombocytopenia (HIT): Initial therapy with warfarin sodium in HIT has resulted in cases of amputation and death. Warfarin sodium may be considered after platelet count has normalized. (5.6)
- Pregnant women with mechanical heart valves: Warfarin sodium may cause fetal harm; however, the benefits may outweigh the risks. (5.7)
ADVERSE REACTIONS
Most common adverse reactions to warfarin sodium are fatal and nonfatal hemorrhage from any tissue or organ. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Exelan Pharmaceuticals, Inc. at 1-866-604-3268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Concomitant use of drugs that increase bleeding risk, antibiotics, antifungals, botanical (herbal) products, and inhibitors and inducers of CYP2C9, 1A2, or 3A4. (7)
- Consult labeling of all concurrently used drugs for complete information about interactions with warfarin sodium or increased risks for bleeding. (7)
USE IN SPECIFIC POPULATIONS
- Pregnant women with mechanical heart valves: Warfarin sodium may cause fetal harm; however, the benefits may outweigh the risks. (8.1)
- Lactation: Monitor breastfeeding infants for bruising or bleeding. (8.2)
- Renal Impairment: Instruct patients with renal impairment to frequently monitor their INR. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: BLEEDING RISK
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Individualized Dosing
2.2 Recommended Target INR Ranges and Durations for Individual Indications
2.3 Initial and Maintenance Dosing
2.4 Monitoring to Achieve Optimal Anticoagulation
2.5 Renal Impairment
2.6 Missed Dose
2.7 Treatment During Dentistry and Surgery
2.8 Conversion From Other Anticoagulants
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Hemorrhage
5.2 Tissue Necrosis
5.3 Calciphylaxis
5.4 Acute Kidney Injury
5.5 Systemic Atheroemboli and Cholesterol Microemboli
5.6 Limb Ischemia, Necrosis, and Gangrene in Patients with HIT and HITTS
5.7 Use in Pregnant Women with Mechanical Heart Valves
5.8 Other Clinical Settings with Increased Risks
5.9 Endogenous Factors Affecting INR
6. ADVERSE REACTIONS
7. DRUG INTERACTIONS
7.1 General Information
7.2 CYP450 Interactions
7.3 Drugs that Increase Bleeding Risk
7.4 Antibiotics and Antifungals
7.5 Botanical (Herbal) Products and Foods
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10. OVERDOSAGE
10.1 Signs and Symptoms
10.2 Treatment
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
14.1 Atrial Fibrillation
14.2 Mechanical and Bioprosthetic Heart Valves
14.3 Myocardial Infarction
15. REFERENCES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: BLEEDING RISK
- Warfarin sodium can cause major or fatal bleeding [see Warnings and Precautions (5.1)].
- Perform regular monitoring of INR in all treated patients [see Dosage and Administration (2.1)].
- Drugs, dietary changes, and other factors affect INR levels achieved with warfarin sodium therapy [see Drug Interactions (7)].
- Instruct patients about prevention measures to minimize risk of bleeding and to report signs and symptoms of bleeding [see Patient Counseling Information (17)].
-
1. INDICATIONS AND USAGE
Warfarin sodium tablets, are indicated for:
- Prophylaxis and treatment of venous thrombosis and its extension, pulmonary embolism (PE).
- Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation (AF) and/or cardiac valve replacement.
- Reduction in the risk of death, recurrent myocardial infarction (MI), and thromboembolic events such as stroke or systemic embolization after myocardial infarction.
Limitations of Use
Warfarin sodium tablets have no direct effect on an established thrombus, nor does it reverse ischemic tissue damage. Once a thrombus has occurred, however, the goals of anticoagulant treatment are to prevent further extension of the formed clot and to prevent secondary thromboembolic complications that may result in serious and possibly fatal sequelae. -
2. DOSAGE AND ADMINISTRATION
2.1 Individualized Dosing
The dosage and administration of warfarin sodium must be individualized for each patient according to the patient’s International Normalized Ratio (INR) response to the drug. Adjust the dose based on the patient’s INR and the condition being treated. Consult the latest evidencebased clinical practice guidelines regarding the duration and intensity of anticoagulation for the indicated conditions.
2.2 Recommended Target INR Ranges and Durations for Individual Indications
An INR of greater than 4.0 appears to provide no additional therapeutic benefit in most patients and is associated with a higher risk of bleeding.
Venous Thromboembolism (including deep venous thrombosis [DVT] and PE)
Adjust the warfarin dose to maintain a target INR of 2.5 (INR range, 2.0 to 3.0) for all treatment durations. The duration of treatment is based on the indication as follows:
- For patients with a DVT or PE secondary to a transient (reversible) risk factor, treatment with warfarin for 3 months is recommended.
- For patients with an unprovoked DVT or PE, treatment with warfarin is recommended for at least 3 months. After 3 months of therapy, evaluate the risk-benefit ratio of long-term treatment for the individual patient.
- For patients with two episodes of unprovoked DVT or PE, long-term treatment with warfarin is recommended. For a patient receiving long-term anticoagulant treatment, periodically reassess the risk-benefit ratio of continuing such treatment in the individual patient.
Atrial Fibrillation
In patients with non-valvular AF, anticoagulate with warfarin to target INR of 2.5 (range, 2.0 to 3.0).
- In patients with non-valvular AF that is persistent or paroxysmal and at high risk of stroke (i.e., having any of the following features: prior ischemic stroke, transient ischemic attack, or systemic embolism, or 2 of the following risk factors: age greater than 75 years, moderately or severely impaired left ventricular systolic function and/or heart failure, history of hypertension, or diabetes mellitus), long-term anticoagulation with warfarin is recommended.
- In patients with non-valvular AF that is persistent or paroxysmal and at an intermediate risk of ischemic stroke (i.e., having 1 of the following risk factors: age greater than 75 years, moderately or severely impaired left ventricular systolic function and/or heart failure, history of hypertension, or diabetes mellitus), long-term anticoagulation with warfarin is recommended.
- For patients with AF and mitral stenosis, long-term anticoagulation with warfarin is recommended.
- For patients with AF and prosthetic heart valves, long-term anticoagulation with warfarin is recommended; the target INR may be increased and aspirin added depending on valve type and position, and on patient factors.
Mechanical and Bioprosthetic Heart Valves
- For patients with a bileaflet mechanical valve or a Medtronic Hall (Minneapolis, MN) tilting disk valve in the aortic position who are in sinus rhythm and without left atrial enlargement, therapy with warfarin to a target INR of 2.5 (range, 2.0 to 3.0) is recommended.
- For patients with tilting disk valves and bileaflet mechanical valves in the mitral position, therapy with warfarin to a target INR of 3.0(range, 2.5 to 3.5) is recommended.
- For patients with caged ball or caged disk valves, therapy with warfarin to a target INR of 3.0 (range, 2.5 to3.5) is recommended.
- For patients with a bioprosthetic valve in the mitral position, therapy with warfarin to a target INR of 2.5 (range, 2.0 to 3.0) for the first 3 months after valve insertion is recommended. If additional risk factors for thromboembolism are present (AF, previous thromboembolism, left ventricular dysfunction), a target INR of 2.5 (range, 2.0 to 3.0) is recommended.
Post-Myocardial Infarction
- For high-risk patients with MI (e.g., those with a large anterior MI, those with significant heart failure, those with intracardiac thrombus visible on transthoracic echocardiography, those with AF, and those with a history of a thromboembolic event), therapy with combined moderate-intensity (INR, 2.0 to 3.0) warfarin plus low-dose aspirin (≤ 100 mg/day) for at least 3 months after the MI is recommended.
Recurrent Systemic Embolism and Other Indications
Oral anticoagulation therapy with warfarin has not been fully evaluated by clinical trials in patients with valvular disease associated with AF, patients with mitral stenosis, and patients with recurrent systemic embolism of unknown etiology. However, a moderate dose regimen (INR 2.0 to 3.0) may be used for these patients.
2.3 Initial and Maintenance Dosing
The appropriate initial dosing of warfarin sodium varies widely for different patients. Not all factors responsible for warfarin dose variability are known, and the initial dose is influenced by:
- Clinical factors including age, race, body weight, sex, concomitant medications, and comorbidities
- Genetic factors (CYP2C9 and VKORC1 genotypes) [see Clinical Pharmacology (12.5)]
Select the initial dose based on the expected maintenance dose, taking into account the above factors. Modify this dose based on consideration of patient-specific clinical factors. Consider lower initial and maintenance doses for elderly and/or debilitated patients and in Asian patients [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3)]. Routine use of loading doses is not recommended as this practice may increase hemorrhagic and other complications and does not offer more rapid protection against clot formation.
Individualize the duration of therapy for each patient. In general, anticoagulant therapy should be continued until the danger of thrombosis and embolism has passed [see Dosage and Administration (2.2)].
Dosing Recommendations without Consideration of Genotype
If the patient’s CYP2C9 and VKORC1 genotypes are not known, the initial dose of warfarin sodium is usually 2 to 5 mg once daily. Determine each patient’s dosing needs by close monitoring of the INR response and consideration of the indication being treated. Typical maintenance doses are 2 to 10 mg once daily.
Dosing Recommendations with Consideration of Genotype
Table 1 displays three ranges of expected maintenance warfarin sodium doses observed in subgroups of patients having different combinations of CYP2C9 and VKORC1 gene variants [see Clinical Pharmacology (12.5)]. If the patient’s CYP2C9 and/or VKORC1 genotype are known, consider these ranges in choosing the initial dose. Patients with CYP2C9 *1/*3, *2/*2, *2/*3, and *3/*3 may require more prolonged time (> 2 to 4 weeks) to achieve maximum INR effect for a given dosage regimen than patients without these CYP variants.
Table 1: Three Ranges of Expected Maintenance Warfarin Sodium Daily Doses Based on CYP2C9 and VKORC1 Genotypes † VKORC1 CYP2C9 †Ranges are derived from multiple published clinical studies. VKORC1 -1639G>A (rs9923231) variant is used in this table. Other co-inherited VKORC1 variants may also be important determinants of warfarin dose. *1/*1 *1/*2 *1/*3 *2/*2 *2/*3 *3/*3 GG 5-7 mg 5-7 mg 3-4 mg 3-4 mg 3-4 mg 0.5-2 mg AG 5-7 mg 3-4 mg 3-4 mg 3-4 mg 0.5-2 mg 0.5-2 mg AA 3-4 mg 3-4 mg 0.5-2 mg 0.5-2 mg 0.5-2 mg 0.5-2 mg 2.4 Monitoring to Achieve Optimal Anticoagulation
Warfarin sodium is a narrow therapeutic range (index) and its action may be affected by factors such as other drugs and dietary vitamin K. Therefore, anticoagulation must be carefully monitored during warfarin sodium therapy. Determine the INR daily after the administration of the initial dose until INR results stabilize in the therapeutic range. After stabilization, maintain dosing within the therapeutic range by performing periodic INRs. The frequency of performing INR should be based on the clinical situation but generally acceptable intervals for INR determinations are 1 to 4 weeks. Perform additional INR tests when other warfarin products are interchanged with warfarin sodium, as well as whenever other medications are initiated, discontinued, or taken irregularly. Heparin, a common concomitant drug, increases the INR [see Dosage and Administration (2.8) and Drug Interactions (7)].
Determinations of whole blood clotting and bleeding times are not effective measures for monitoring of warfarin sodium therapy.
2.5 Renal Impairment
No dosage adjustment is necessary for patients with renal failure. Monitor INR more frequently in patients with compromised renal function to maintain INR within the therapeutic range [see Warnings and Precautions (5.4) and Use in Specific Populations (8.6)].
2.6 Missed Dose
The anticoagulant effect of warfarin sodium persists beyond 24 hours. If a patient misses a dose of warfarin sodium at the intended time of day, the patient should take the dose as soon as possible on the same day. The patient should not double the dose the next day to make up for a missed dose.
2.7 Treatment During Dentistry and Surgery
Some dental or surgical procedures may necessitate the interruption or change in the dose of warfarin sodium therapy. Consider the benefits and risks when discontinuing warfarin sodium even for a short period of time. Determine the INR immediately prior to any dental or surgical procedure. In patients undergoing minimally invasive procedures who must be anticoagulated prior to, during, or immediately following these procedures, adjusting the dosage of warfarin sodium to maintain the INR at the low end of the therapeutic range may safely allow for continued anticoagulation.
2.8 Conversion From Other Anticoagulants
Heparin
Since the full anticoagulant effect of warfarin sodium is not achieved for several days, heparin is preferred for initial rapid anticoagulation. During initial therapy with warfarin sodium, the interference with heparin anticoagulation is of minimal clinical significance. Conversion to warfarin sodium may begin concomitantly with heparin therapy or may be delayed 3 to 6 days.
To ensure therapeutic anticoagulation, continue full dose heparin therapy and overlap warfarin sodium therapy with heparin for 4 to 5 days and until warfarin sodium has produced the desired therapeutic response as determined by INR, at which point heparin may be discontinued.
As heparin may affect the INR, patients receiving both heparin and warfarin sodium should have INR monitoring at least:
- 5 hours after the last intravenous bolus dose of heparin, or
- 4 hours after cessation of a continuous intravenous infusion of heparin, or
- 24 hours after the last subcutaneous heparin injection.
Warfarin sodium may increase the activated partial thromboplastin time (aPTT) test, even in the absence of heparin. A severe elevation (> 50 seconds) in aPTT with an INR in the desired range has been identified as an indication of increased risk of postoperative hemorrhage.
Other Anticoagulants
Consult the labeling of other anticoagulants for instructions on conversion to warfarin sodium.
-
3. DOSAGE FORMS AND STRENGTHS
Warfarin sodium tablets, USP are supplied as follows:
- 1 mg Tablets: Light pink, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘1’ on the bottom of other side.
- 2 mg Tablets: Lavender, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘2’ on the bottom of other side.
- 2.5 mg Tablets: Green, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘2 1/ 2’ on the bottom of other side.
- 3 mg Tablets: Tan, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘3’ on the bottom of other side.
- 4 mg Tablets: Blue, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘4’ on the bottom of other side.
- 5 mg Tablets: Peach, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘5’ on the bottom of other side.
- 6 mg Tablets: Teal, Round, Flat Beveled edge tablets de-bossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘6’ on the bottom of other side.
- 7.5 mg Tablets: Yellow, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘7 1/ 2’ on the bottom of other side.
- 10 mg Tablets: White, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘10’ on the bottom of other side.
-
4. CONTRAINDICATIONS
Warfarin Sodium is contraindicated in
- Pregnancy
Warfarin sodium tablets are contraindicated in women who are pregnant except in pregnant women with mechanical heart valves, who are at high risk of thromboembolism [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)]. Warfarin sodium tablets can cause fetal harm when administered to a pregnant woman. Warfarin sodium tablets exposure during pregnancy causes a recognized pattern of major congenital malformations (warfarin embryopathy and fetotoxicity), fatal fetal hemorrhage, and an increased risk of spontaneous abortion and fetal mortality. If warfarin sodium tablets are used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations (8.1)].
Warfarin Sodium is contraindicated in patients with:- Hemorrhagic tendencies or blood dyscrasias
- Recent or contemplated surgery of the central nervous system or eye, or traumatic surgery resulting in large open surfaces [see Warnings and Precautions (5.8)]
- Bleeding tendencies associated with:
− Active ulceration or overt bleeding of the gastrointestinal, genitourinary, or respiratory tract
− Central nervous system hemorrhage
− Cerebral aneurysms, dissecting aorta
− Pericarditis and pericardial effusions
− Bacterial endocarditis
- Threatened abortion, eclampsia, and preeclampsia
- Unsupervised patients with conditions associated with potential high level of non-compliance
- Spinal puncture and other diagnostic or therapeutic procedures with potential for uncontrollable bleeding
- Hypersensitivity to warfarin or to any other components of this product (e.g., anaphylaxis) [see Adverse Reactions (6)]
- Major regional or lumbar block anesthesia
- Malignant hypertension
-
5. WARNINGS AND PRECAUTIONS
5.1 Hemorrhage
Warfarin sodium can cause major or fatal bleeding. Bleeding is more likely to occur within the first month. Risk factors for bleeding include high intensity of anticoagulation (INR > 4.0), age greater than or equal to 65, history of highly variable INRs, history of gastrointestinal bleeding, hypertension, cerebrovascular disease, anemia, malignancy, trauma, renal impairment, certain genetic factors [see Clinical Pharmacology (12.5)], certain concomitant drugs [see Drug Interactions (7)], and long duration of warfarin therapy.
Perform regular monitoring of INR in all treated patients. Those at high risk of bleeding may benefit from more frequent INR monitoring, careful dose adjustment to desired INR, and a shortest duration of therapy appropriate for the clinical condition. However, maintenance of INR in the therapeutic range does not eliminate the risk of bleeding.
Drugs, dietary changes, and other factors affect INR levels achieved with warfarin sodium therapy. Perform more frequent INR monitoring when starting or stopping other drugs, including botanicals, or when changing dosages of other drugs [see Drug Interactions (7)].
Instruct patients about prevention measures to minimize risk of bleeding and to report signs and symptoms of bleeding [see Patient Counseling Information (17)].
5.2 Tissue Necrosis
Warfarin sodium can cause necrosis and/or gangrene of skin and other tissues, which is an uncommon but serious risk (< 0.1%). Necrosis may be associated with local thrombosis and usually appears within a few days of the start of warfarin sodium therapy. In severe cases of necrosis, treatment through debridement or amputation of the affected tissue, limb, breast, or penis has been reported.
Careful clinical evaluation is required to determine whether necrosis is caused by an underlying disease. Although various treatments have been attempted, no treatment for necrosis has been considered uniformly effective. Discontinue warfarin sodium therapy if necrosis occurs. Consider alternative drugs if continued anticoagulation therapy is necessary.
5.3 Calciphylaxis
Warfarin sodium can cause fatal and serious calciphylaxis or calcium uremic arteriolopathy, which has been reported in patients with and without end-stage renal disease. When calciphylaxis is diagnosed in these patients, discontinue Warfarin sodium and treat calciphylaxis as appropriate. Consider alternative anticoagulation therapy.
5.4 Acute Kidney Injury
In patients with altered glomerular integrity or with a history of kidney disease, acute kidney injury may occur with Warfarin sodium, possibly in relation to episodes of excessive anticoagulation and hematuria [see Use in Specific Populations (8.6)]. More frequent monitoring of anticoagulation is advised in patients with compromised renal function.
5.5 Systemic Atheroemboli and Cholesterol Microemboli
Anticoagulation therapy with warfarin sodium may enhance the release of atheromatous plaque emboli. Systemic atheroemboli and cholesterol microemboli can present with a variety of signs and symptoms depending on the site of embolization. The most commonly involved visceral organs are the kidneys followed by the pancreas, spleen, and liver. Some cases have progressed to necrosis or death. A distinct syndrome resulting from microemboli to the feet is known as “purple toes syndrome.” Discontinue warfarin sodium therapy if such phenomena are observed. Consider alternative drugs if continued anticoagulation therapy is necessary.
5.6 Limb Ischemia, Necrosis, and Gangrene in Patients with HIT and HITTS
Do not use warfarin sodium as initial therapy in patients with heparin-induced thrombocytopenia (HIT) and with heparin-induced thrombocytopenia with thrombosis syndrome (HITTS). Cases of limb ischemia, necrosis, and gangrene have occurred in patients with HIT and HITTS when heparin treatment was discontinued and warfarin therapy was started or continued. In some patients, sequelae have included amputation of the involved area and/or death. Treatment with warfarin sodium may be considered after the platelet count has normalized.
5.7 Use in Pregnant Women with Mechanical Heart Valves
Warfarin sodium can cause fetal harm when administered to a pregnant woman. While warfarin sodium is contraindicated during pregnancy, the potential benefits of using warfarin sodium may outweigh the risks for pregnant women with mechanical heart valves at high risk of thromboembolism. In those individual situations, the decision to initiate or continue warfarin sodium should be reviewed with the patient, taking into consideration the specific risks and benefits pertaining to the individual patient’s medical situation, as well as the most current medical guidelines. Warfarin sodium exposure during pregnancy causes a recognized pattern of major congenital malformations (warfarin embryopathy and fetotoxicity), fatal fetal hemorrhage, and an increased risk of spontaneous abortion and fetal mortality. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations (8.1)].
5.8 Other Clinical Settings with Increased Risks
In the following clinical settings, the risks of warfarin sodium therapy may be increased:
- Moderate to severe hepatic impairment
- Infectious diseases or disturbances of intestinal flora (e.g., sprue, antibiotic therapy)
- Use of an indwelling catheter
- Severe to moderate hypertension
- Deficiency in protein C-mediated anticoagulant response: Warfarin sodium reduces the synthesis of the naturally occurring anticoagulants, protein C and protein S. Hereditary or acquired deficiencies of protein C or its cofactor, protein S, have been associated with tissue necrosis following warfarin administration. Concomitant anticoagulation therapy with heparin for 5 to 7 days during initiation of therapy with warfarin sodium may minimize the incidence of tissue necrosis in these patients.
- Eye surgery: In cataract surgery, warfarin sodium use was associated with a significant increase in minor complications of sharp needle and local anesthesia block but not associated with potentially sight-threatening operative hemorrhagic complications. As warfarin sodium cessation or reduction may lead to serious thromboembolic complications, the decision to discontinue warfarin sodium before a relatively less invasive and complex eye surgery, such as lens surgery, should be based upon the risks of anticoagulant therapy weighed against the benefits.
- Polycythemia vera
- Vasculitis
- Diabetes mellitus
5.9 Endogenous Factors Affecting INR
The following factors may be responsible for increased INR response: diarrhea, hepatic disorders, poor nutritional state, steatorrhea, or vitamin K deficiency.
The following factors may be responsible for decreased INR response: increased vitamin K intake or hereditary warfarin resistance.
-
6. ADVERSE REACTIONS
The following serious adverse reactions to warfarin sodium are discussed in greater detail in other sections of the labeling:
- Hemorrhage [see Boxed Warning, Warnings and Precautions (5.1), and Overdosage (10)]
- Tissue Necrosis [see Warnings and Precautions (5.2)]
- Calciphylaxis [see Warnings and Precautions (5.3)]
- Acute Kidney Injury [see Warnings and Precautions (5.4)]
- Systemic atheroemboli and cholesterol microemboli [see Warnings and Precautions (5.5)]
- Limb Ischemia, Necrosis, and Gangrene in Patients with HIT and HITTS [see Warnings and Precautions (5.6)]
- Other Clinical Settings with Increased Risks [see Warnings and Precautions (5.8)]
Other adverse reactions to warfarin sodium include:
- Immune system disorders: hypersensitivity/allergic reactions (including urticaria and anaphylactic reactions)
- Vascular disorders: vasculitis
- Hepatobiliary disorders: hepatitis, elevated liver enzymes. Cholestatic hepatitis has been associated with concomitant administration of warfarin sodium and ticlopidine.
- Gastrointestinal disorders: nausea, vomiting, diarrhea, taste perversion, abdominal pain, flatulence, bloating
- Skin disorders: rash, dermatitis (including bullous eruptions), pruritus, alopecia
- Respiratory disorders: tracheal or tracheobronchial calcification
- General disorders: chills
-
7. DRUG INTERACTIONS
7.1 General Information
Drugs may interact with warfarin sodium through pharmacodynamic or pharmacokinetic mechanisms. Pharmacodynamic mechanisms for drug interactions with warfarin sodium are synergism (impaired hemostasis, reduced clotting factor synthesis), competitive antagonism (vitamin K), and alteration of the physiologic control loop for vitamin K metabolism (hereditary resistance). Pharmacokinetic mechanisms for drug interactions with warfarin sodium are mainly enzyme induction, enzyme inhibition, and reduced plasma protein binding. It is important to note that some drugs may interact by more than one mechanism.
More frequent INR monitoring should be performed when starting or stopping other drugs, including botanicals, or when changing dosages of other drugs, including drugs intended for short-term use (e.g., antibiotics, antifungals, corticosteroids) [see Boxed Warning].
Consult the labeling of all concurrently used drugs to obtain further information about interactions with warfarin sodium or adverse reactions pertaining to bleeding.
7.2 CYP450 Interactions
CYP450 isozymes involved in the metabolism of warfarin include CYP2C9, 2C19, 2C8, 2C18, 1A2, and 3A4. The more potent warfarin S-enantiomer is metabolized by CYP2C9 while the R-enantiomer is metabolized by CYP1A2 and 3A4.
- Inhibitors of CYP2C9, 1A2, and/or 3A4 have the potential to increase the effect (increase INR) of warfarin by increasing the exposure of warfarin.
- Inducers of CYP2C9, 1A2, and/or 3A4 have the potential to decrease the effect (decrease INR) of warfarin by decreasing the exposure of warfarin.
Examples of inhibitors and inducers of CYP2C9, 1A2, and 3A4 are below in Table 2; however, this list should not be considered all-inclusive. Consult the labeling of all concurrently used drugs to obtain further information about CYP450 interaction potential. The CYP450 inhibition and induction potential should be considered when starting, stopping, or changing dose of concomitant mediations. Closely monitor INR if a concomitant drug is a CYP2C9, 1A2, and/or 3A4 inhibitor or inducer.
Table 2: Examples of CYP450 Interactions with Warfarin Enzyme Inhibitors Inducers CYP2C9 amiodarone, capecitabine, cotrimoxazole, etravirine, fluconazole, fluvastatin, fluvoxamine, metronidazole, miconazole, oxandrolone, sulfinpyrazone, tigecycline, voriconazole, zafirlukast aprepitant, bosentan, carbamazepine, phenobarbital, rifampin CYP1A2 acyclovir, allopurinol, caffeine, cimetidine, ciprofloxacin, disulfiram, enoxacin, famotidine, fluvoxamine, methoxsalen, mexiletine, norfloxacin, oral contraceptives, phenylpropanolamine, propafenone, propranolol, terbinafine, thiabendazole, ticlopidine, verapamil, zileuton montelukast, moricizine, omeprazole, phenobarbital, phenytoin, cigarette smoking CYP3A4 alprazolam, amiodarone, amlodipine, amprenavir, aprepitant, atorvastatin, atazanavir, bicalutamide, cilostazol, cimetidine, ciprofloxacin, clarithromycin, conivaptan, cyclosporine, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fluoxetine, fluvoxamine, fosamprenavir, imatinib, indinavir, isoniazid, itraconazole, ketoconazole, lopinavir/ritonavir, nefazodone, nelfinavir, nilotinib, oral contraceptives, posaconazole, ranitidine, ranolazine, ritonavir, saquinavir, telithromycin, tipranavir, voriconazole, zileuton armodafinil, amprenavir, aprepitant, bosentan, carbamazepine, efavirenz, etravirine, modafinil, nafcillin, phenytoin, pioglitazone, prednisone, rifampin, rufinamide 7.3 Drugs that Increase Bleeding Risk
Examples of drugs known to increase the risk of bleeding are presented in Table 3. Because bleeding risk is increased when these drugs are used concomitantly with warfarin, closely monitor patients receiving any such drug with warfarin.
Table 3: Drugs that Can Increase the Risk of Bleeding Drug Class Specific Drugs Anticoagulants argatroban, dabigatran, bivalirudin, desirudin, heparin, lepirudin Antiplatelet Agents aspirin, cilostazol, clopidogrel, dipyridamole, prasugrel, ticlopidine Nonsteroidal Anti-Inflammatory Agents celecoxib, diclofenac, diflunisal, fenoprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, mefenamic acid, naproxen, oxaprozin, piroxicam, sulindac Serotonin Reuptake Inhibitors citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, paroxetine, sertraline, venlafaxine, vilazodone. 7.4 Antibiotics and Antifungals
There have been reports of changes in INR in patients taking warfarin and antibiotics or antifungals, but clinical pharmacokinetic studies have not shown consistent effects of these agents on plasma concentrations of warfarin.
Closely monitor INR when starting or stopping any antibiotic or antifungal in patients taking warfarin.
7.5 Botanical (Herbal) Products and Foods
More frequent INR monitoring should be performed when starting or stopping botanicals.
Few adequate, well-controlled studies evaluating the potential for metabolic and/or pharmacologic interactions between botanicals and warfarin sodium exist. Due to a lack of manufacturing standardization with botanical medicinal preparations, the amount of active ingredients may vary. This could further confound the ability to assess potential interactions and effects on anticoagulation.
Some botanicals may cause bleeding events when taken alone (e.g., garlic and Ginkgo biloba) and may have anticoagulant, antiplatelet, and/or fibrinolytic properties. These effects would be expected to be additive to the anticoagulant effects of warfarin sodium. Conversely, some botanicals may decrease the effects of warfarin sodium (e.g., co-enzyme Q 10, St. John’s wort, ginseng). Some botanicals and foods can interact with warfarin sodium through CYP450 interactions (e.g., echinacea, grapefruit juice, ginkgo, goldenseal, St. John’s wort).
The amount of vitamin K in food may affect therapy with warfarin sodium. Advise patients taking warfarin sodium to eat a normal, balanced diet maintaining a consistent amount of vitamin K. Patients taking warfarin sodium should avoid drastic changes in dietary habits, such as eating large amounts of green leafy vegetables.
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Warfarin sodium is contraindicated in women who are pregnant except in pregnant women with mechanical heart valves, who are at high risk of thromboembolism, and for whom the benefits of warfarin sodium may outweigh the risks [ see Warnings and Precautions (5.7)]. Warfarin sodium can cause fetal harm. Exposure to warfarin during the first trimester of pregnancy caused a pattern of congenital malformations in about 5% of exposed offspring. Because these data were not collected in adequate and well-controlled studies, this incidence of major birth defects is not an adequate basis for comparison to the estimated incidences in the control group or the U.S. general population and may not reflect the incidences observed in practice. Consider the benefits and risks of warfarin sodium and possible risks to the fetus when prescribing warfarin sodium to a pregnant woman.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.Clinical Considerations
Fetal/Neonatal Adverse Reactions
In humans, warfarin crosses the placenta, and concentrations in fetal plasma approach the maternal values. Exposure to warfarin during the first trimester of pregnancy caused a pattern of congenital malformations in about 5% of exposed offspring. Warfarin embryopathy is characterized by nasal hypoplasia with or without stippled epiphyses (chondrodysplasia punctata) and growth retardation (including low birth weight). Central nervous system and eye abnormalities have also been reported, including dorsal midline dysplasia characterized by agenesis of the corpus callosum, Dandy-Walker malformation, midline cerebellar atrophy, and ventral midline dysplasia characterized by optic atrophy. Mental retardation, blindness, schizencephaly, microcephaly, hydrocephalus, and other adverse pregnancy outcomes have been reported following warfarin exposure during the second and third trimesters of pregnancy [see Contraindications (4)].
8.2 Lactation
Risk Summary
Warfarin was not present in human milk from mothers treated with warfarin from a limited published study. Because of the potential for serious adverse reactions, including bleeding in a breastfed infant, consider the developmental and health benefits of breastfeeding along with the mother’s clinical need for warfarin sodium and any potential adverse effects on the breastfed infant from warfarin sodium or from the underlying maternal condition before prescribing warfarin sodium to a lactating woman.
Clinical Considerations
Monitor breastfeeding infants for bruising or bleeding.
Data
Human Data
Based on published data in 15 nursing mothers, warfarin was not detected in human milk. Among the 15 full-term newborns, 6 nursing infants had documented prothrombin times within the expected range. Prothrombin times were not obtained for the other 9 nursing infants. Effects in premature infants have not been evaluated.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Warfarin sodium can cause fetal harm [see Use in Specific Populations (8.1)] .
Verify the pregnancy status of females of reproductive potential prior to initiating warfarin sodium therapy.
ContraceptionFemales
Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the final dose of warfarin sodium.
8.4 Pediatric Use
Adequate and well-controlled studies with warfarin sodium have not been conducted in any pediatric population, and the optimum dosing, safety, and efficacy in pediatric patients is unknown. Pediatric use of warfarin sodium is based on adult data and recommendations, and available limited pediatric data from observational studies and patient registries. Pediatric patients administered warfarin sodium should avoid any activity or sport that may result in traumatic injury.
The developing hemostatic system in infants and children results in a changing physiology of thrombosis and response to anticoagulants. Dosing of warfarin in the pediatric population varies by patient age, with infants generally having the highest, and adolescents having the lowest milligram per kilogram dose requirements to maintain target INRs. Because of changing warfarin requirements due to age, concomitant medications, diet, and existing medical condition, target INR ranges may be difficult to achieve and maintain in pediatric patients, and more frequent INR determinations are recommended. Bleeding rates varied by patient population and clinical care center in pediatric observational studies and patient registries.
Infants and children receiving vitamin K-supplemented nutrition, including infant formulas, may be resistant to warfarin therapy, while human milk-fed infants may be sensitive to warfarin therapy.
8.5 Geriatric Use
Of the total number of patients receiving warfarin sodium in controlled clinical trials for which data were available for analysis, 1885 patients (24.4%) were 65 years and older, while 185 patients (2.4%) were 75 years and older. No overall differences in effectiveness or safety were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Patients 60 years or older appear to exhibit greater than expected INR response to the anticoagulant effects of warfarin [see Clinical Pharmacology (12.3)]. Warfarin sodium tablets are contraindicated in any unsupervised patient with senility. Conduct more frequent monitoring for bleeding with administration of warfarin sodium to elderly patients in any situation or with any physical condition where added risk of hemorrhage is present. Consider lower initiation and maintenance doses of warfarin sodium in elderly patients [see Dosage and Administration (2.2, 2.3)].
8.6 Renal Impairment
Renal clearance is considered to be a minor determinant of anticoagulant response to warfarin. No dosage adjustment is necessary for patients with renal impairment.
Instruct patients with renal impairment taking warfarin to monitor their INR more frequently [see Warnings and Precautions (5.4)].
-
10. OVERDOSAGE
10.1 Signs and Symptoms
Bleeding (e.g., appearance of blood in stools or urine, hematuria, excessive menstrual bleeding, melena, petechiae, excessive bruising or persistent oozing from superficial injuries, unexplained fall in hemoglobin) is a manifestation of excessive anticoagulation.
10.2 Treatment
The treatment of excessive anticoagulation is based on the level of the INR, the presence or absence of bleeding, and clinical circumstances. Reversal of warfarin sodium anticoagulation may be obtained by discontinuing warfarin sodium therapy and, if necessary, by administration of oral or parenteral vitamin K 1.
The use of vitamin K 1 reduces response to subsequent warfarin sodium therapy and patients may return to a pretreatment thrombotic status following the rapid reversal of a prolonged INR. Resumption of warfarin sodium administration reverses the effect of vitamin K, and a therapeutic INR can again be obtained by careful dosage adjustment. If rapid re-anticoagulation is indicated, heparin may be preferable for initial therapy.
Prothrombin complex concentrate (PCC), fresh frozen plasma, or activated Factor VII treatment may be considered if the requirement to reverse the effects of warfarin sodium is urgent. A risk of hepatitis and other viral diseases is associated with the use of blood products; PCC and activated Factor VII are also associated with an increased risk of thrombosis. Therefore, these preparations should be used only in exceptional or life-threatening bleeding episodes secondary to warfarin sodium overdosage.
-
11. DESCRIPTION
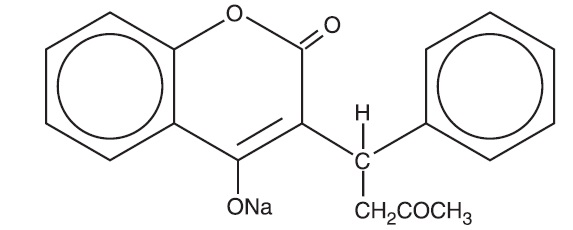
Warfarin sodium, USP is an anticoagulant that acts by inhibiting vitamin K-dependent coagulation factors. The chemical name of warfarin sodium is 3-(α- acetonylbenzyl)-4-hydroxycoumarin sodium salt, which is a racemic mixture of the R- and S-enantiomers. Crystalline warfarin sodium is an isopropanol clathrate. Its empirical formula is C 19H 15NaO 4, and its structural formula is represented by the following:
Crystalline warfarin sodium occurs as a white, odorless, crystalline powder that is discolored by light. It is very soluble in water, freely soluble in alcohol, and very slightly soluble in chloroform and ether.
Each warfarin sodium tablet, USP intended for oral administration contains warfarin sodium clathrates equivalent to 1 mg or 2 mg or 2.5 mg or 3 mg or 4 mg or 5 mg or 6 mg or 7.5 mg or 10 mg of warfarin sodium. In addition each tablet contains the inactive ingredients lactose monohydrate, starch, pregelatinized starch, hydroxypropyl cellulose, starlac and magnesium stearate. Additionally each
1 mg tablet contains: D&C Red #30 aluminum lake
2 mg tablet contains: FD&C Red #40 aluminum lake and FD&C Blue#2
2.5 mg tablet contains: D&C Yellow # 10 aluminum lake and FD&C Blue #2
3 mg tablet contains: FD&C Yellow # 6 aluminum lake, FD&C Blue#2 and FD&C Red # 40 aluminum lake
4 mg tablet contains: FD&C Blue#2
5 mg tablet contains: FD&C Yellow # 6 aluminum lake
6 mg tablet contains: FD&C Yellow # 6 aluminum lake and FD&C Blue #2
7.5 mg tablet contains: D&C Yellow # 10 aluminum lake and FD&C Yellow # 6 aluminum lake
10 mg tablet is dye free
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Warfarin acts by inhibiting the synthesis of vitamin K-dependent clotting factors, which include Factors II, VII, IX, and X, and the anticoagulant proteins C and S. Vitamin K is an essential cofactor for the post ribosomal synthesis of the vitamin K-dependent clotting factors. Vitamin K promotes the biosynthesis of γ -carboxyglutamic acid residues in the proteins that are essential for biological activity. Warfarin is thought to interfere with clotting factor synthesis by inhibition of the C1 subunit of vitamin K epoxide reductase (VKORC1) enzyme complex, thereby reducing the regeneration of vitamin K 1 epoxide [see Clinical Pharmacology (12.5)].
12.2 Pharmacodynamics
An anticoagulation effect generally occurs within 24 hours after warfarin administration. However, peak anticoagulant effect may be delayed 72 to 96 hours. The duration of action of a single dose of racemic warfarin is 2 to 5 days. The effects of warfarin sodium may become more pronounced as effects of daily maintenance doses overlap. This is consistent with the half-lives of the affected vitamin K-dependent clotting factors and anticoagulation proteins: Factor II - 60 hours, VII - 4 to 6 hours, IX - 24 hours, X - 48 to 72 hours, and proteins C and S are approximately 8 hours and 30 hours, respectively.
12.3 Pharmacokinetics
Warfarin sodium is a racemic mixture of the R- and S-enantiomers of warfarin. The S-enantiomer exhibits 2 to 5 times more anticoagulant activity than the R-enantiomer in humans, but generally has a more rapid clearance.
Absorption
Warfarin is essentially completely absorbed after oral administration, with peak concentration generally attained within the first four hours.
Distribution
Warfarin shows a volume of distribution of about 0.14 L/kg. Approximately 99% of the drug is bound to plasma proteins.
Metabolism
The elimination of warfarin is almost entirely by metabolism. Warfarin is stereoselectively metabolized by hepatic cytochrome P-450 (CYP450) microsomal enzymes to inactive hydroxylated metabolites (predominant route) and by reductases to reduced metabolites (warfarin alcohols) with minimal anticoagulant activity. Identified metabolites of warfarin include dehydrowarfarin, two diastereoisomer alcohols, and 4′-, 6-, 7-, 8-, and 10-hydroxywarfarin. The CYP450 isozymes involved in the metabolism of warfarin include CYP2C9, 2C19, 2C8, 2C18, 1A2, and 3A4. CYP2C9, a polymorphic enzyme, is likely to be the principal form of human liver CYP450 that modulates the in vivo anticoagulant activity of warfarin. Patients with one or more variant CYP2C9 alleles have decreased S-warfarin clearance [see Clinical Pharmacology (12.5)].
Excretion
The terminal half-life of warfarin after a single dose is approximately one week; however, the effective half-life ranges from 20 to 60 hours, with a mean of about 40 hours. The clearance of R-warfarin is generally half that of S-warfarin, thus as the volumes of distribution are similar, the half-life of R-warfarin is longer than that of S-warfarin. The half-life of R-warfarin ranges from 37 to 89 hours, while that of S-warfarin ranges from 21 to 43 hours. Studies with radio labeled drug have demonstrated that up to 92% of the orally administered dose is recovered in urine. Very little warfarin is excreted unchanged in urine. Urinary excretion is in the form of metabolites.
Geriatric Patients
Patients 60 years or older appear to exhibit greater than expected INR response to the anticoagulant effects of warfarin. The cause of the increased sensitivity to the anticoagulant effects of warfarin in this age group is unknown but may be due to a combination of pharmacokinetic and pharmacodynamic factors. Limited information suggests there is no difference in the clearance of S-warfarin; however, there may be a slight decrease in the clearance of R-warfarin in the elderly as compared to the young. Therefore, as patient age increases, a lower dose of warfarin is usually required to produce a therapeutic level of anticoagulation [see Dosage and Administration (2.3, 2.4)].
Asian Patients
Asian patients may require lower initiation and maintenance doses of warfarin. A non-controlled study of 151 Chinese outpatients stabilized on warfarin for various indications reported a mean daily warfarin requirement of 3.3 ± 1.4 mg to achieve an INR of 2 to 2.5. Patient age was the most important determinant of warfarin requirement in these patients, with a progressively lower warfarin requirement with increasing age.
12.5 Pharmacogenomics
CYP2C9 and VKORC1 Polymorphisms
The S-enantiomer of warfarin is mainly metabolized to 7-hydroxywarfarin by CYP2C9, a polymorphic enzyme. The variant alleles, CYP2C9*2 and CYP2C9*3, result in decreased in vitro CYP2C9 enzymatic 7-hydroxylation of S-warfarin. The frequencies of these alleles in Caucasians are approximately 11% and 7% for CYP2C9*2 and CYP2C9*3, respectively.
Other CYP2C9 alleles associated with reduced enzymatic activity occur at lower frequencies, including *5, *6, and *11 alleles in populations of African ancestry and *5, *9, and *11 alleles in Caucasians.
Warfarin reduces the regeneration of vitamin K from vitamin K epoxide in the vitamin K cycle through inhibition of VKOR, a multiprotein enzyme complex. Certain single nucleotide polymorphisms in the VKORC1 gene (e.g., –1639G>A) have been associated with variable warfarin dose requirements. VKORC1 and CYP2C9 gene variants generally explain the largest proportion of known variability in warfarin dose requirements.
CYP2C9 and VKORC1 genotype information, when available, can assist in selection of the initial dose of warfarin [see Dosage and Administration (2.3)].
- 13. NONCLINICAL TOXICOLOGY
-
14. CLINICAL STUDIES
14.1 Atrial Fibrillation
In five prospective, randomized, controlled clinical trials involving 3,711 patients with non-rheumatic AF, warfarin significantly reduced the risk of systemic thromboembolism including stroke (see Table 4). The risk reduction ranged from 60% to 86% in all except one trial (CAFA: 45%), which was stopped early due to published positive results from two of these trials. The incidence of major bleeding in these trials ranged from 0.6% to 2.7% (see Table 4).
Table 4: Clinical Studies of Warfarin in Non-Rheumatic AF Patients* N Thromboembolism % Major Bleeding Study Warfarin-
Treated PatientsControl
PatientsPT Ratio INR % Risk
Reductionp-value Warfarin-
Treated PatientsControl
PatientsAFASAK 335 336 1.5 to 2.0 2.8 to 4.2 60 0.027 0.6 0.0 SPAF 210 211 1.3 to 1.8 2.0 to 4.5 67 0.01 1.9 1.9 BAATAF 212 208 1.2 to 1.5 1.5 to 2.7 86 <0.05 0.9 0.5 CAFA 187 191 1.3 to 1.6 2.0 to 3.0 45 0.25 2.7 0.5 SPINAF 260 265 1.2 to 1.5 1.4 to 2.8 79 0.001 2.3 1.5 * All study results of Warfarin vs. control are based on intention-to-treat analysis and include ischemic stroke and systemic thromboembolism, excluding hemorrhagic stroke and transient ischemic attacks.
Trials in patients with both AF and mitral stenosis suggest a benefit from anticoagulation with warfarin [see Dosage and Administration (2.2)].
14.2 Mechanical and Bioprosthetic Heart Valves
In a prospective, randomized, open-label, positive-controlled study in 254 patients with mechanical prosthetic heart valves, the thromboembolic-free interval was found to be significantly greater in patients treated with warfarin alone compared with dipyridamole/aspirin-treated patients (p<0.005) and pentoxifylline/aspirin-treated patients (p<0.05). The results of this study are presented in Table 5.
Table 5: Prospective, Randomized, Open-Label, Positive-Controlled Clinical Study of Warfarin in Patients with Mechanical Prosthetic Heart Valves Patients Treated With py=patient years Event Warfarin Dipyridamole/Aspirin Pentoxifylline/Aspirin Thromboembolism 2.2/100 py 8.6/100 py 7.9/100 py Major Bleeding 2.5/100 py 0.0/100 py 0.9/100 py In a prospective, open-label, clinical study comparing moderate (INR 2.65) vs. high intensity (INR 9.0) warfarin therapies in 258 patients with mechanical prosthetic heart valves, thromboembolism occurred with similar frequency in the two groups (4.0 and 3.7 events per 100 patient years, respectively). Major bleeding was more common in the high intensity group. The results of this study are presented in Table 6.
Table 6: Prospective, Open-Label Clinical Study of Warfarin in Patients with Mechanical Prosthetic Heart Valves Event Moderate Warfarin Therapy
INR 2.65High Intensity Warfarin Therapy
INR 9.0py=patient years Thromboembolism 4.0/100 py 3.7/100 py Major Bleeding 0.95/100 py 2.1/100 py In a randomized trial in 210 patients comparing two intensities of warfarin therapy (INR 2.0 to 2.25 vs. INR 2.5 to 4.0) for a three month period following tissue heart valve replacement, thromboembolism occurred with similar frequency in the two groups (major embolic events 2.0 % vs. 1.9 %, respectively, and minor embolic events 10.8% vs. 10.2%, respectively). Major hemorrhages occurred in 4.6% of patients in the higher intensity INR group compared to zero in the lower intensity INR group.
14.3 Myocardial Infarction
WARIS (The Warfarin Re-Infarction Study) was a double-blind, randomized study of 1214 patients 2 to 4 weeks post-infarction treated with warfarin to a target INR of 2.8 to 4.8. The primary endpoint was a composite of total mortality and recurrent infarction. A secondary endpoint of cerebrovascular events was assessed. Mean follow-up of the patients was 37 months. The results for each endpoint separately, including an analysis of vascular death, are provided in Table 7:
The results for each endpoint separately, including an analysis of vascular death, are provided in Table 7:
Table 7: WARIS – Endpoint Analysis of Separate Events % Risk Warfarin Placebo Reduction Event (N=607) (N=607) RR (95% CI) ( p-value) RR=Relative risk; Risk reduction=(1-RR); CI=Confidence interval; MI=Myocardial infarction; py=patient years Total Patient Years of Follow-up 2018 1944 Total Mortality 94 (4.7/100 py) 123 (6.3/100 py) 0.76 (0.60, 0.97) 24 (p=0.030) Vascular Death 82 (4.1/100 py) 105 (5.4/100 py) 0.78 (0.60, 1.02) 22 (p=0.068) Recurrent MI 82 (4.1/100 py) 124 (6.4/100 py) 0.66 (0.51, 0.85) 34 (p=0.001) Cerebrovascular Event 20 (1.0/100 py) 44 (2.3/100 py) 0.46 (0.28, 0.75) 54 (p=0.002) WARIS II (The Warfarin, Aspirin, Re-Infarction Study) was an open-label, randomized study of 3630 patients hospitalized for acute myocardial infarction treated with warfarin to a target INR 2.8 to 4.2, aspirin 160 mg per day, or warfarin to a target INR 2.0 to 2.5 plus aspirin 75 mg per day prior to hospital discharge. The primary endpoint was a composite of death, nonfatal reinfarction, or thromboembolic stroke. The mean duration of observation was approximately 4 years. The results for WARIS II are provided in the Table 8.
Table 8: WARIS II – Distribution of Events According to Treatment Group Event Aspirin
(N=1206)Warfarin
(N=1216)Aspirin plus
Warfarin
(N=1208)Rate Ratio
(95% CI)p-value a Major bleeding episodes were defined as nonfatal cerebral hemorrhage or bleeding necessitating surgical intervention or blood transfusion. b The rate ratio is for aspirin plus warfarin as compared with aspirin. c The rate ratio is for warfarin as compared with aspirin. d Minor bleeding episodes were defined as non-cerebral hemorrhage not necessitating surgical intervention or blood transfusion. e Includes death, nonfatal reinfarction, and thromboembolic cerebral stroke. CI=confidence interval ND=not determined No. of Events Major Bleeding a 8 33 28 3.35 b (ND)
4.00 c (ND)ND
NDMinor Bleeding d 39 103 133 3.21 b (ND)
2.55 c (ND)ND
NDComposite Endpoints e 241 203 181 0.81 (0.69-0.95) b
0.71 (0.60-0.83) c0.03
0.001Reinfarction 117 90 69 0.56 (0.41-0.78) b
0.74 (0.55-0.98) c<0.001
0.03Thromboembolic Stroke 32 17 17 0.52 (0.28-0.98) b
0.52 (0.28-0.97) c0.03
0.03Death 92 96 95 0.82 There were approximately four times as many major bleeding episodes in the two groups receiving warfarin than in the group receiving aspirin alone. Major bleeding episodes were not more frequent among patients receiving aspirin plus warfarin than among those receiving warfarin alone, but the incidence of minor bleeding episodes was higher in the combined therapy group.
- 15. REFERENCES
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Warfarin sodium tablets, USP are supplied as follows:
1 mg Tablets: Light pink, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘1’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-341-01) and 1000’s count (NDC: 51407-341-10)
2 mg Tablets: Lavender, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘2’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-342-01) and 1000’s count (NDC: 51407-342-10)
2.5 mg Tablets: Green, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘2 1/ 2’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-343-01) and 1000’s count (NDC: 51407-343-10)
3 mg Tablets: Tan, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘3’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-344-01) and 1000’s count (NDC: 51407-344-10)
4 mg Tablets: Blue, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘4’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-345-01) and 1000’s count (NDC: 51407-345-10)
5 mg Tablets: Peach, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘5’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-346-01) and 1000’s count (NDC: 51407-346-10)
6 mg Tablets: Teal, Round, Flat Beveled edge tablets de-bossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘6’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-347-01) and 1000’s count (NDC: 51407-347-10)
7.5 mg Tablets: Yellow, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘7 1/ 2’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-348-01)
10 mg Tablets: White, Round, Flat Beveled edge tablets debossed ‘I’ on the left side of bisect and ‘G’ on the right side of bisect on one side and ‘W’ on the top and ‘10’ on the bottom of other side, supplied in bottles of 100’s count (NDC: 51407-349-01)
Storage: Store at 20° to 25°C (68° F to 77° F). [see USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP.
Special Handling
Procedures for proper handling and disposal of potentially hazardous drugs should be considered. Guidelines on this subject have been published [see References (15)].Pharmacy and clinical personnel who are pregnant should avoid exposure to crushed or broken tablets [see Use in Specific Populations (8.1)] .
-
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Medication Guide).
Instructions for Patients
Advise patients to:
- Strictly adhere to the prescribed dosage schedule [see Dosage and Administration (2.1)].
- If the prescribed dose of warfarin sodium is missed, take the dose as soon as possible on the same day but do not take a double dose of warfarin sodium the next day to make up for missed doses [see Dosage and Administration (2.6)].
- Obtain prothrombin time tests and make regular visits to their physician or clinic to monitor therapy [see Dosage and Administration (2.1)].
- Be aware that if therapy with warfarin sodium is discontinued, the anticoagulant effects of warfarin sodium may persist for about 2 to 5 days [see Clinical Pharmacology (12.2)].
- Avoid any activity or sport that may result in traumatic injury [see Use in Specific Populations (8.4)]. And to tell their physician if they fall often as this may increase their risk for complications.
- Eat a normal, balanced diet to maintain a consistent intake of vitamin K. Avoid drastic changes in dietary habits, such as eating large amounts of leafy, green vegetables [see Drug Interactions (7.5)].
- Contact their physician to report any serious illness, such as severe diarrhea, infection, or fever [see Warnings and Precautions (5) and Adverse Reactions (6)].
- Immediately contact their physician when experiencing pain and discoloration of the skin (a purple bruise like rash) mostly on areas of the body with a high fat content, such as breasts, thighs, buttocks, hips and abdomen [see Warnings and Precautions (5.2)].
- Immediately contact their physician when experiencing any unusual symptom or pain since Warfarin sodium may cause small cholesterol or athero emboli. On feet it may appear as a sudden cool, painful, purple discoloration of toe(s) or forefoot [see Warnings and Precautions (5.5)].
- Immediately contact their physician when taking Warfarin sodium after any heparin formulation therapy and experiencing bloody or black stools or appearance of bruises, or bleeding [see Warnings and Precautions (5.6)].
- To tell all of their healthcare professionals and dentists that they are taking Warfarin sodium. This should be done before they have any surgery or dental procedure [see Dosage and Administration (2.7)].
- Carry identification stating that they are taking warfarin sodium.
Bleeding Risks
Advise patients to:
- Notify their physician immediately if any unusual bleeding or symptoms occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness [see Box Warning and Warnings and Precautions (5.1)].
Concomitant Medications and Botanicals (Herbals)
Advise patients to:
- Not take or discontinue any other drug, including salicylates (e.g., aspirin and topical analgesics), other over-the-counter drugs, and botanical (herbal) products except on advice of your physician [see Drug Interactions (7)].
Pregnancy and Nursing
Advise patients to:
- Notify their physician if they are pregnant or planning to become pregnant or considering breast feeding [see Use in Specific Populations (8.1, 8.2, 8.3)].
- Avoid warfarin sodium during pregnancy except in pregnant women with mechanical heart valves, who are at risk of thromboembolism [see Contraindications (4)]. Use effective measures to avoid pregnancy while taking warfarin sodium. This is very important because their unborn baby could be seriously harmed if they take warfarin sodium while they are pregnant [see Use in Specific Populations (8.1, 8.3)].
Revised: 10/2019
-
MEDICATION GUIDE
Warfarin Sodium Tablets, USP
(war' far in soe' dee um)What is the most important information I should know about warfarin sodium?
Warfarin sodium can cause bleeding which can be serious and sometimes lead to death. This is because warfarin sodium is a blood thinner medicine that lowers the chance of blood clots forming in your body.
- You may have a higher risk of bleeding if you take warfarin sodium and:
- are 65 years of age or older
- have a history of stomach or intestinal bleeding
- have high blood pressure (hypertension)
- have a history of stroke, or “mini-stroke” (transient ischemic attack or TIA)
- have serious heart disease
- have a low blood count or cancer
- have had trauma, such as an accident or surgery
- have kidney problems
- take other medicines that increase your risk of bleeding, including:
- a medicine that contains heparin
- other medicines to prevent or treat blood clots
- nonsteroidal anti-inflammatory drugs (NSAIDs)
- take warfarin sodium for a long time. Warfarin sodium, is the active ingredient in warfarin sodium tablets.
Tell your healthcare provider if you take any of these medicines. Ask your healthcare provider if you are not sure if your medicine is one listed above.
Many other medicines can interact with warfarin sodium and affect the dose you need or increase warfarin sodium side effects. Do not change or stop any of your medicines or start any new medicines before you talk to your healthcare provider.
Do not take other medicines that contain warfarin sodium while taking warfarin sodium tablets.
- Get your regular blood test to check for your response to warfarin sodium. This blood test is called an INR test. The INR test checks to see how fast your blood clots. Your healthcare provider will decide what INR numbers are best for you. Your dose of warfarin sodium will be adjusted to keep your INR in a target range for you.
- Call your healthcare provider right away if you get any of the following signs or symptoms of bleeding problems:
- pain, swelling, or discomfort
- headaches, dizziness, or weakness
- unusual bruising (bruises that develop without known cause or grow in size)
- nosebleeds
- bleeding gums
- bleeding from cuts takes a long time to stop
- menstrual bleeding or vaginal bleeding that is heavier than normal
- pink or brown urine
- red or black stools
- coughing up blood
- vomiting blood or material that looks like coffee grounds
- Some foods and beverages can interact with warfarin sodium and affect your treatment and dose.
- Eat a normal, balanced diet. Talk to your healthcare provider before you make any diet changes. Do not eat large amounts of leafy, green vegetables. Leafy, green vegetables contain vitamin K. Certain vegetable oils also contain large amounts of vitamin K. Too much vitamin K can lower the effect of warfarin sodium.
- Always tell all of your healthcare providers that you take warfarin sodium.
- Wear or carry information that you take warfarin sodium.
See “What are the possible side effects of warfarin sodium?” for more information about side effects.
What is warfarin sodium?
Warfarin sodium is prescription medicine used to treat blood clots and to lower the chance of blood clots forming in your body. Blood clots can cause a stroke, heart attack, or other serious conditions if they form in the legs or lungs.
Who should not take warfarin sodium?
Do not take warfarin sodium if:
- your risk of having bleeding problems is higher than the possible benefit of treatment. Your healthcare provider will decide if warfarin sodium is right for you.
- you are pregnant unless you have a mechanical heart valve. Warfarin sodium may cause birth defects, miscarriage, or death of your unborn baby.
- you are allergic to warfarin or any of the other ingredients in warfarin sodium tablets. See the end of this leaflet for a complete list of ingredients in warfarin sodium tablets.
Before taking Warfarin sodium, tell your healthcare provider about all of your medical conditions, including if you:
- have bleeding problems
- fall often
- have liver problems
- have kidney problems or are undergoing dialysis
- have high blood pressure
- have a heart problem called congestive heart failure
- have diabetes
- plan to have any surgery or a dental procedure
- are pregnant or plan to become pregnant. See “ Who should not take warfarin sodium?”
- Your health care provider will do a pregnancy test before you start treatment with warfarin sodium. Females who can become pregnant should use effective birth control during treatment, and for at least 1 month after the last dose of warfarin sodium.
- are breastfeeding. You and your health care provider should decide if you will take warfarin sodium and breastfeed. Check your baby for bruising or bleeding if you take warfarin sodium and breastfeed.
Tell all of your healthcare providers and dentists that you are taking warfarin sodium. They should talk to the healthcare provider who prescribed warfarin sodium for you before you have any surgery or dental procedure. Your warfarin sodium may need to be stopped for a short time or you may need your dose adjusted.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some of your other medicines may affect the way warfarin sodium works. Certain medicines may increase your risk of bleeding. See “ What is the most important information I should know about warfarin sodium?”
How should I take warfarin sodium?
- Take warfarin sodium exactly as prescribed. Your healthcare provider will adjust your dose from time to time depending on your response to warfarin sodium.
- You must have regular blood tests and visits with your healthcare provider to monitor your condition.
- If you miss a dose of warfarin sodium, call your healthcare provider. Take the dose as soon as possible on the same day. Do not take a double dose of warfarin sodium the next day to make up for a missed dose.
- Call your healthcare provider right away if you:
- take too much warfarin sodium
- are sick with diarrhea, an infection, or have a fever
- fall or injure yourself, especially if you hit your head. Your healthcare provider may need to check you
What should I avoid while taking warfarin sodium?
- Do not do any activity or sport that may cause a serious injury.
What are the possible side effects of warfarin sodium?
Warfarin sodium may cause serious side effects, including:
- See “ What is the most important information I should know about warfarin sodium?”
- Death of skin tissue (skin necrosis or gangrene). This can happen soon after starting warfarin sodium. It happens because blood clots form and block blood flow to an area of your body. Call your healthcare provider right away if you have pain, color, or temperature change to any area of your body. You may need medical care right away to prevent death or loss (amputation) of your affected body part.
- Kidney problems. Kidney injury may happen in people who take Warfarin sodium. Tell your healthcare provider right away if you develop blood in your urine. Your healthcare provider may do tests more often during treatment with Warfarin sodium to check for bleeding if you already have kidney problems.
- “Purple toes syndrome.” Call your healthcare provider right away if you have pain in your toes and they look purple in color or dark in color.
These are not all of the side effects of warfarin sodium. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store warfarin sodium?
- Store warfarin sodium tablets at 20°C to 25°C (68°F to 77°F).
- Keep warfarin sodium tablets in a tightly closed container.
- Keep warfarin sodium tablets out of the light and moisture.
- Follow your healthcare provider or pharmacist instructions about the right way to throw away outdated or unused warfarin sodium tablets.
- Females who are pregnant should not handle crushed or broken warfarin sodium tablets.
Keep warfarin sodium tablets and all medicines out of the reach of children.
General information about the safe and effective use of warfarin sodium.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use warfarin sodium for a condition for which it was not prescribed. Do not give warfarin sodium to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about warfarin sodium. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about warfarin sodium that is written for health professionals.
If you would like more information, talk to your healthcare provider or call Exelan Pharmaceuticals, Inc. at 1-866-604-3268.
What are the ingredients in warfarin sodium tablets, USP?
Active ingredient: Warfarin Sodium, USP
Inactive ingredients: Lactose monohydrate, starch, pregelatinized starch, hydroxypropyl cellulose, starlac and magnesium stearate. Additionally each:
1 mg tablet contains: D&C Red #30 aluminum lake
2 mg tablet contains: FD&C Red #40 aluminum lake and FD&C Blue#2
2.5 mg tablet contains: D&C Yellow # 10 aluminum lake and FD&C Blue#2
3 mg tablet contains: FD&C Yellow # 6 aluminum lake, FD&C Blue#2 and FD&C Red # 40 aluminum lake
4 mg tablet contains: FD&C Blue#2
5 mg tablet contains: FD&C Yellow # 6 aluminum lake
6 mg tablet contains: FD&C Yellow # 6 aluminum lake and FD&C Blue #2
7.5 mg tablet contains: D&C Yellow # 10 aluminum lake and FD&C Yellow # 6 aluminum lake
10 mg tablet is dye free.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Exelan Pharmaceuticals, Inc.
Lawrenceville, GA 30046
Manufactured By:
InvaGen Pharmaceuticals, Inc.
(a sunsidiary of Cipla Ltd.)
Hauppauge, NY 11788
Revised: 10/2019
Marketed by:
GSMS, Inc.
Camarillo, CA USA 93012
Rx only
- You may have a higher risk of bleeding if you take warfarin sodium and:
-
PRINCIPAL DISPLAY PANEL
NDC: 51407-341-01
Warfarin Sodium Tablets, USP
Crystalline * 1 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-342-01
Warfarin Sodium Tablets, USP
Crystalline * 2 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-343-01
Warfarin Sodium Tablets, USP
Crystalline * 2.5 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-344-01
Warfarin Sodium Tablets, USP
Crystalline * 3 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-345-01
Warfarin Sodium Tablets, USP
Crystalline * 4 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets
 NDC: 51407-346-01
NDC: 51407-346-01
Warfarin Sodium Tablets, USP
Crystalline * 5 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-347-01
Warfarin Sodium Tablets, USP
Crystalline * 6 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-348-01
Warfarin Sodium Tablets, USP
Crystalline * 7.5 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

NDC: 51407-349-01
Warfarin Sodium Tablets, USP
Crystalline * 10 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx Only 100 Tablets

-
INGREDIENTS AND APPEARANCE
WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-341(NDC:76282-327) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 1 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C RED NO. 30 (UNII: 2S42T2808B) Product Characteristics Color pink (Light pink) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-341-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-341-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-342(NDC:76282-328) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 2 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color purple (Lavender) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-342-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-342-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-343(NDC:76282-329) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 2.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color green Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;2;1;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-343-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-343-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-344(NDC:76282-330) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 3 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color brown (Tan) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-344-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-344-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-345(NDC:76282-331) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 4 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-345-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-345-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-346(NDC:76282-332) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange (Peach) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-346-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-346-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-347(NDC:76282-333) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 6 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color turquoise (Teal) Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-347-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 2 NDC: 51407-347-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-348(NDC:76282-334) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 7.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;7;1;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-348-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 WARFARIN SODIUM
warfarin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51407-349(NDC:76282-335) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WARFARIN SODIUM (UNII: 6153CWM0CL) (WARFARIN - UNII:5Q7ZVV76EI) WARFARIN SODIUM 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code IG;W;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51407-349-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090935 05/25/2011 Labeler - Golden State Medical Supply (603184490) Establishment Name Address ID/FEI Business Operations Golden State Medical Supply 603184490 relabel(51407-341, 51407-342, 51407-343, 51407-344, 51407-345, 51407-346, 51407-347, 51407-348, 51407-349)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.