Tussin by QUALITY CHOICE (Chain Drug Marketing Association) Drug Facts
Tussin by
Drug Labeling and Warnings
Tussin by is a Otc medication manufactured, distributed, or labeled by QUALITY CHOICE (Chain Drug Marketing Association). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TUSSIN NON DROWSY- guaifenesin liquid
QUALITY CHOICE (Chain Drug Marketing Association)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
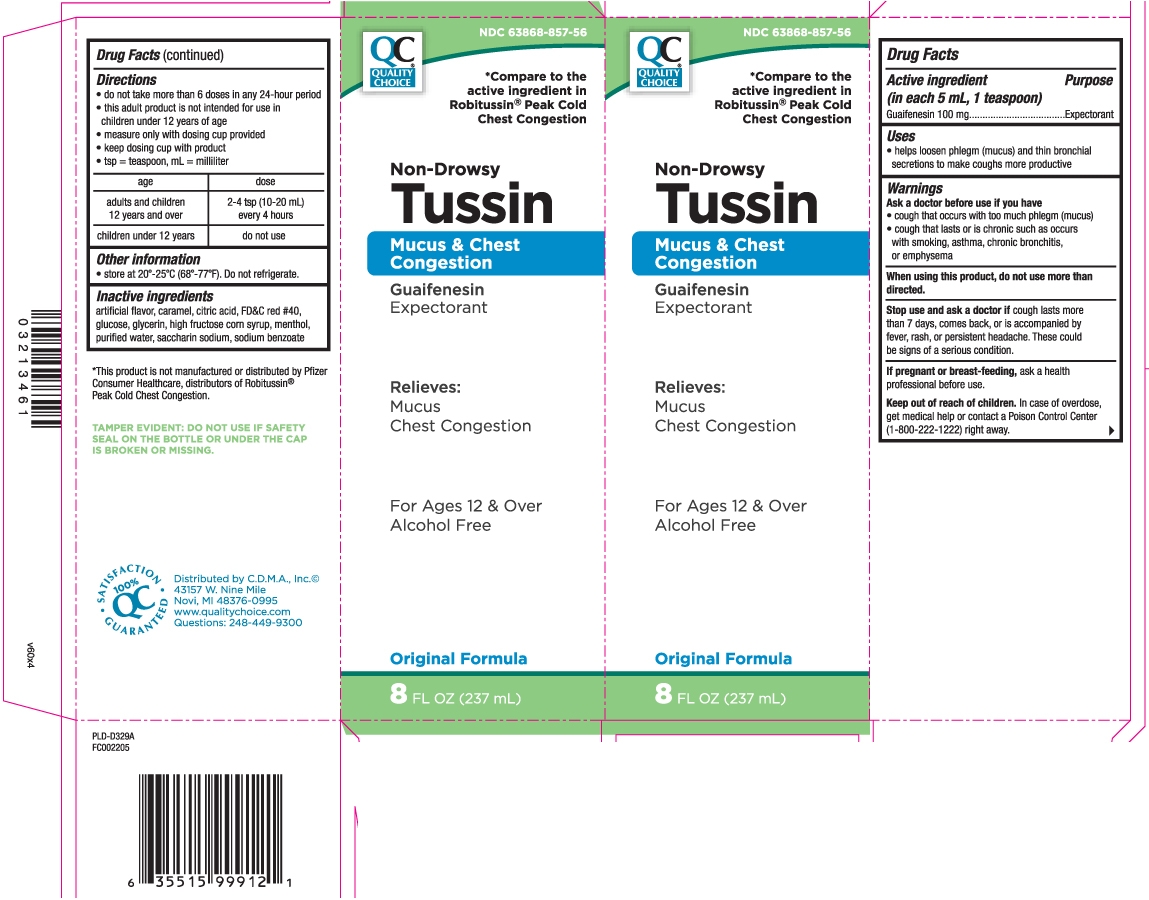
Drug Facts
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- this adult product is not intended for use in children under 12 years of age
- measure only with dosing cup provided
- keep dosing cup with product
- tsp = teaspoon, mL = milliliter
| age | dose |
| adults and children 12 years and over | 2-4 tsp (10-20 mL) every 4 hours |
| children under 12 years | do not use |
Inactive ingredients
artificial flavor, caramel, citric acid, FD&C red #40, glucose, glycerin, high fructose corn syrup, menthol, purified water, saccharin sodium, sodium benzoate
Principal Display Panel
*Compare to the active ingredients in Robitussin® Peak Cold Chest Congestion
Non-Drowsy
Tussin
Mucus & Chest Congestion
Guaifenesin Expectorant
Relieves:
Mucus
Chest congestion
For Ages 12 & Over
Alcohol Free
DM Formula
FL OZ (mL)
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Robitussin® Peak Cold Chest Congestion.
TAMPER EVIDENT: DO NOT USE IF SAFETY SEAL ON THE BOTTLE OR UNDER THE CAP IS BROKEN OR MISSING.
Distributed by C.D.M.A., Inc.©
43157 W. Nine Mile
Novi, MI 48376-0995
www.qualitychoice.com
Questions: 248-449-9300
| TUSSIN
NON DROWSY
guaifenesin liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774) |