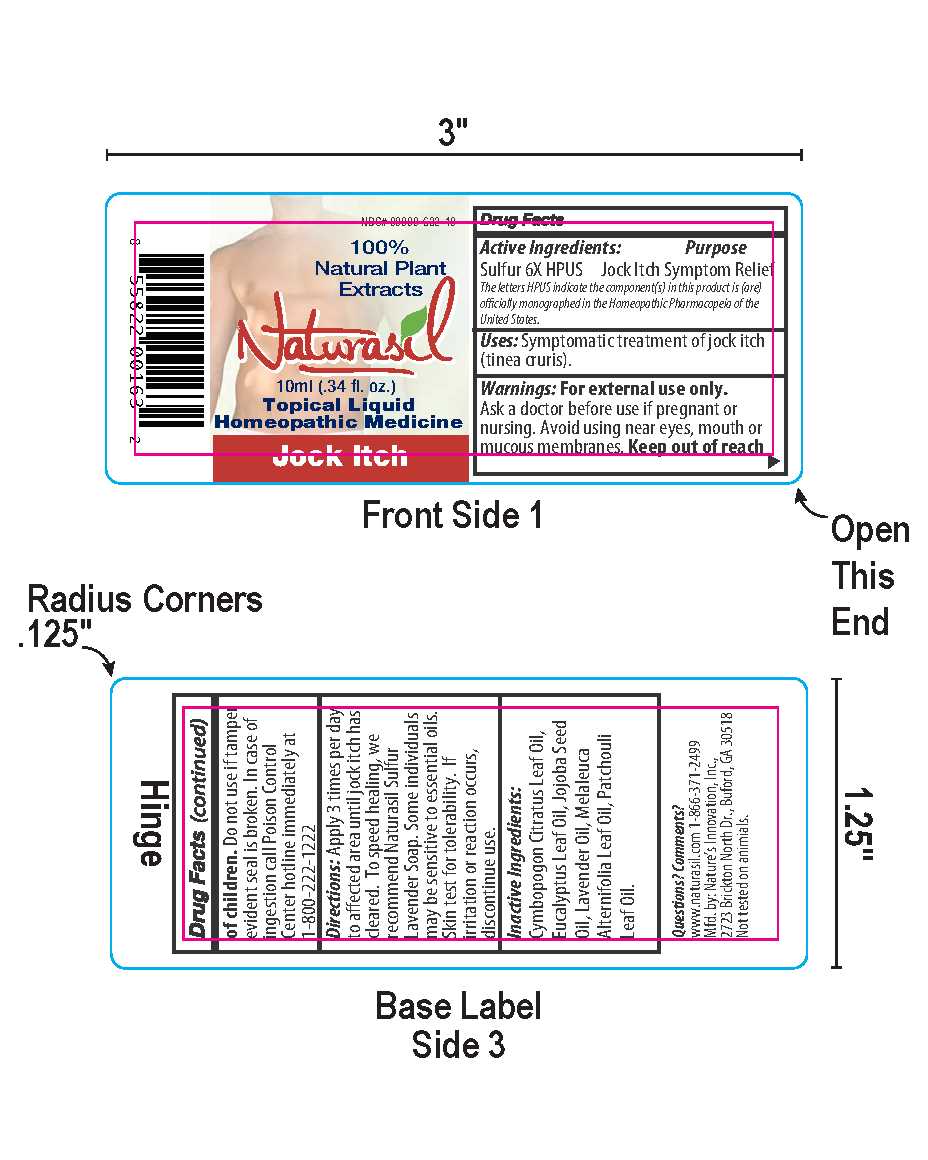
Naturasil by Nature's Innovation, Inc. Jock Itch 10ml Label
Naturasil by
Drug Labeling and Warnings
Naturasil by is a Homeopathic medication manufactured, distributed, or labeled by Nature's Innovation, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NATURASIL JOCK ITCH- sulfur liquid
Nature's Innovation, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Jock Itch 10ml Label
Directions
Directions: Apply 3 times per day to affected area until jock itch has cleared. To speed healing, we recommend Naturasil Sulfur Lavender Soap. Some individuals may be sensitive to essential oils. Skin test for tolerability. If irritation or reaction occurs, discontinue use.
Inactive Ingredients
Inactive Ingredients:Cymbopogon Citratus Leaf Oil, Eucalyptus Leaf Oil, Jojoba Seed Oil, Lavender Oil, Melaleuca Alternifolia Leaf Oil, Patchouli Leaf Oil.
| NATURASIL
JOCK ITCH
sulfur liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Nature's Innovation, Inc. (602969854) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nature's Innovation, Inc. | 602969854 | manufacture(10893-632) | |
Trademark Results [Naturasil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NATURASIL 85461535 4216710 Live/Registered |
Nature's Innovation, Inc. 2011-11-01 |
 NATURASIL 85461495 4216709 Live/Registered |
Nature's Innovation, Inc. 2011-11-01 |
 NATURASIL 78739288 not registered Dead/Abandoned |
Trask Research, Inc. 2005-10-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.