alcohol by Magic Blue, LLC / Creative Essences Inc Hand Sanitizer
alcohol by
Drug Labeling and Warnings
alcohol by is a Otc medication manufactured, distributed, or labeled by Magic Blue, LLC, Creative Essences Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
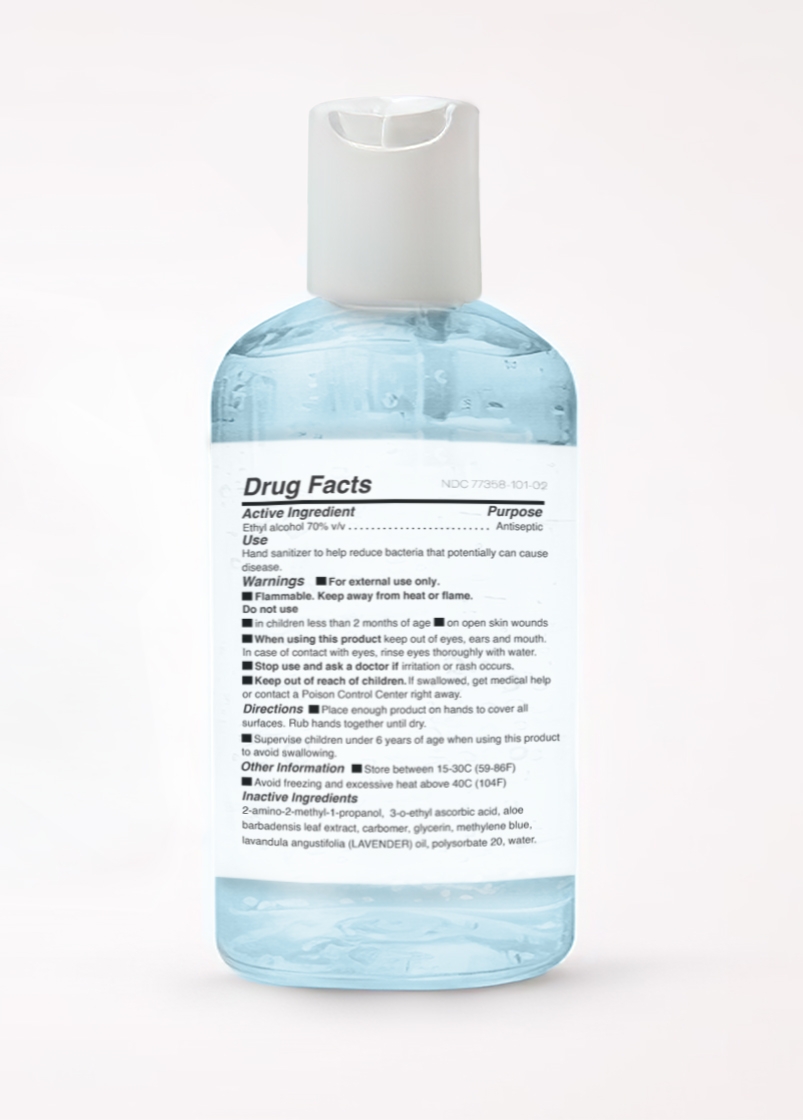
ALCOHOL- bluelene moisturizing hand sanitizer gel
Magic Blue, LLC
----------
Hand Sanitizer
Hand Sanitizer travel Size
2-amino-2-methyl-1-propanol, 3-o-ethyl ascorbic acid, aloe barbadensis leaf extract,carbomer, glycerin, methylene blue, lavendula angustifolia oil (LAVENDER),polysorbate 20, water.
Hand Sanitizer Travel Size
When using this product keep out of eyes, ears, mouth. In case od contact with eyes, rinse yes thoroughly with water.
Hand Sanitizer Travel Size
Keep out of reach of children, If swallowed, get medical help or contact a Poison Control Center right away.
Hand Sanitizer Travel Size
Directions: Place enough product on hands to cover all surfaces. Rub hands together until dry. Supervise children under 6 years of age when using this product to avoid swallowing.
| ALCOHOL
bluelene moisturizing hand sanitizer gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Magic Blue, LLC (114903371) |
| Registrant - Magic Blue, LLC (114903371) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Creative Essences Inc | 079120182 | MANUFACTURE(77358-101) | |
Trademark Results [alcohol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALCOHOL 85863688 4414391 Live/Registered |
Leopardi & Shrum Acquisitions, Inc. 2013-02-28 |
 ALCOHOL 75750120 2405710 Dead/Cancelled |
Piser, John 1999-08-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.